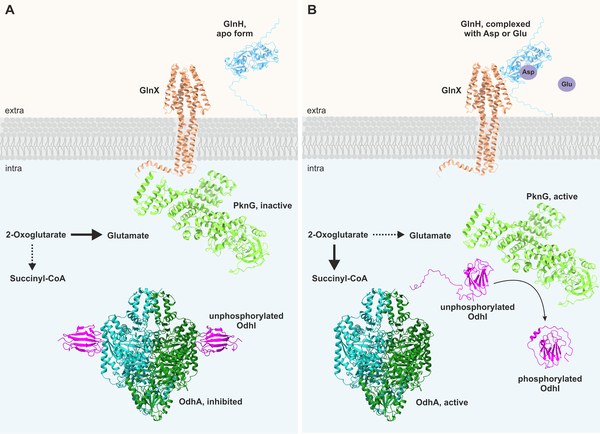
Upcycling von Kunststoffen
Es gibt immer mehr Mikroben und Enzyme, die widerspenstige Kunststoffe wie PET und Polyurethan abbauen können. Diese können im Rahmen eines Bio-Upcycling-Konzepts eingesetzt werden: Kunststoffabfälle werden als Kohlenstoffquelle für die Biotechnologie genutzt und so in eine breite Palette von Chemikalien mit hohem Mehrwert umgewandelt.