Surprising Arrangement for Common Liquid Crystal
16. April 2012
Spotlights on Recent JACS Publications
The particles in liquid crystals arrange themselves based on interparticle interactions, creating various structures somewhere between the total disorder found in a liquid and the rigid order of a packed three-dimensional crystal. Increasing the concentration of particles shifts the liquid crystal structure into different phases dictated by the location and orientation of the particles.
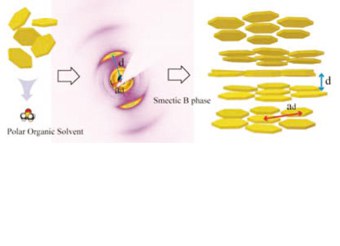
In water, flakes of aluminum hydroxide, or gibbsite, form two concentration-controlled liquid crystalline phases, nematic and columnar, due to the particle anisotropic shape and particle interactions governed by ions in the water. When Dzina
Kleshchanok and co-workers changed the solvent in a gibbsite liquid crystal, they found a surprising particle arrangement: the ordered layers of a phase called smectic B (DOI: 10.1021/ ja300527w). The polar, yet non-ionizable, solvent dimethyl sulfoxide (DMSO) stabilizes long-range charge repulsion between the positively charged hexagonal flakes, causing the particles to organize in this unexpected fashion. Until now, only rod-shaped particles and molecules were known to assemble into this phase, and computer models did not predict that flakelike particles could form this structure. Now scientists can shift between the structures of known liquid crystal phases, and even introduce new phases, by finetuning the particle interactions. Creating a particular structure on demand is important for controlling the shape of the growing polymer by templates from the liquid crystals.
Melissae Fellet, Ph.D.
Lyotropic Smectic B Phase Formed in Suspensions of ChargedColloidal Platelets
