Alzheimer’s research: Article in Chemical Science
Düsseldorf / Jülich, June 27 2018 – Oligomers are aggregates made up of a small number of protein molecules. In the case of Alzheimer's disease, for example, this is the protein amyloid beta (Aβ). Apart from oligomers, larger structures can also develop out of these proteins, i.e. fibrils and ultimately plaques, which form from fibrils. All these substances are toxic to nerve cells. It is already known that the oligomers are the most dangerous, since even in very small quantities they damage or even kill off nerve cells.
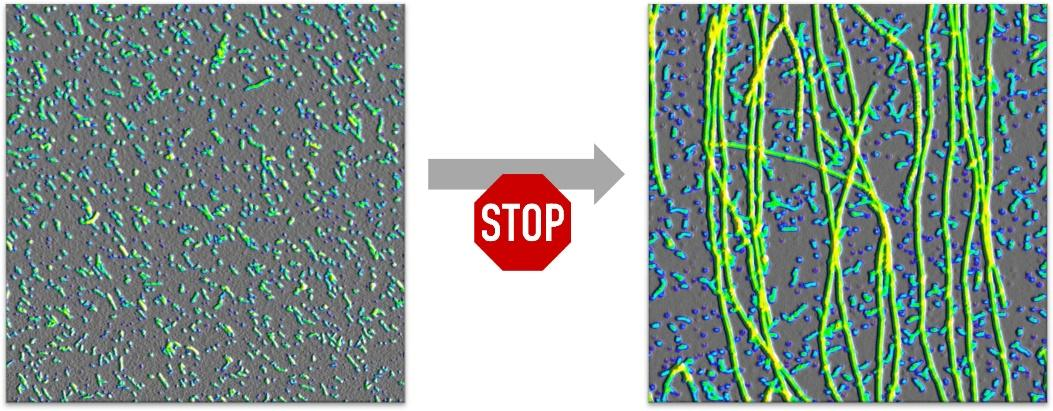
Together with colleagues from the University of South Florida, the researchers in Düsseldorf and Jülich led by Dr. Wolfgang Hoyer of HHU's Institute of Physical Biology examined how oligomers develop and how they are disassembled over the course of time to form fibrils. It emerged that oligomers and fibrils form independently of each other and both compete for the same supply of amyloid beta building blocks. Although the oligomers are indeed more short-lived than the fibrils and disintegrate after a certain time into their individual components, the researchers found out that they actively disrupt fibril formation and are thus able to protect themselves from disassembly.
"Our results explain why the severity of Alzheimer's disease depends only minimally on the number of plaques. There is a far greater correlation between the clinical symptoms and the number of oligomers," says Dr. Hoyer, expounding the far-reaching consequences of the study. This would mean that the oligomers are also a prime target when developing drugs to treat Alzheimer's disease.
Original publication:
Filip Hasecke, Tatiana Miti, Carlos Perez, Jeremy Barton, Daniel Schölzel, Lothar Gremer, Clara S. R. Grüning, Garrett Matthews, Georg Meisl, Tuomas P. J. Knowles, Dieter Willbold, Philipp Neudecker, Henrike Heise, Ghanim Ullah, Wolfgang Hoyer and Martin Muschol, Origin of metastable oligomers and their effects on amyloid fibril self-assembly, Chemical Science, 13.06.2018. DOI: 10.1039/c8sc01479e
