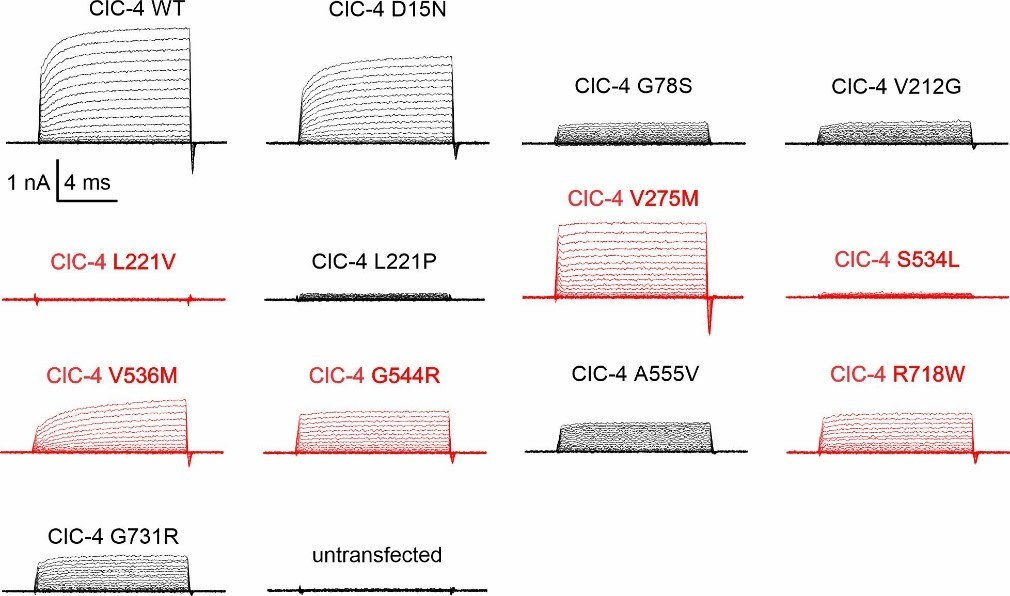
Lin X., He H., Macias-Mendoza M., Longo P., Bungert-Plumke S., Franzen A., Zhou S., Zhu S., Li M., Ye y., Tian X., Li H., Wang Y., Luo R., Miteff C., Goel H., Machtens LP., Peng J., Guzman R.E. (2025). Novel CLCN3 variants expand the clinical phenotype of the CLCN3-related condition and implicate impaired ion transport and ClC-3/TMEM9 interaction in pathogenesis. (In submission)
Balduin, C., Qi, G., Schoneck, M., Trinkel, V., Schemmert, S., Guzman, G. A., Bungert-Plumke, S., Klussendorf, M., Neumaier, B., Feldmeyer, D., Shah, N. J., Stauber, T., Langen, K. J., Guzman, R. E. & Willuweit, A. (2025). Disruption Of Clc-3-Mediated 2cl(-)/H(+) Exchange Leads To Behavioural Deficits And Thalamic Atrophy. Sci Rep, 15, 33326. https://doi.org/10.1038/s41598-025-19757-2
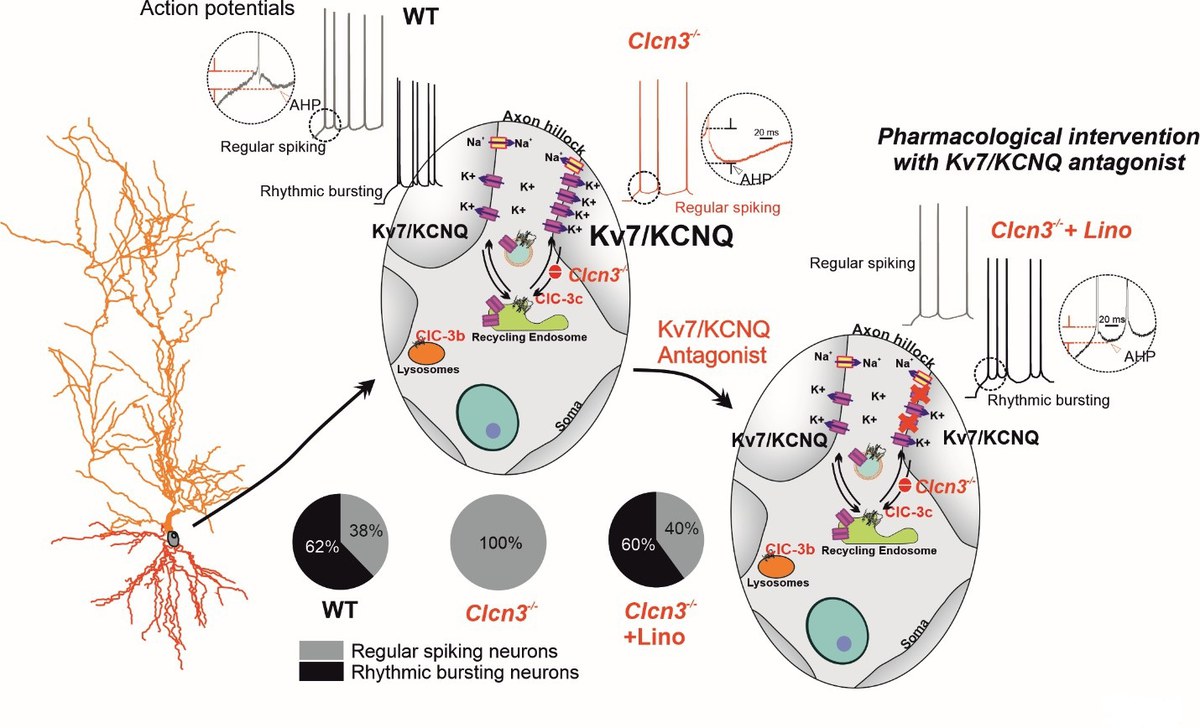
Qi G., Diaz-Castillo A., Aretzweiler C., Steinmetz L., Bungert-Plümke S., Müller F., Feldmeyer D., Guzman R.E. (2025). Endosomal 2Cl-/H+ exchangers regulate neuronal excitability by tuning Kv7/KCNQ channel density. Brain, awaf243, https://doi.org/10.1093/brain/awaf243. DPG (German Physiological Society) – Paper of the Month November 2025
Sahly AN, Sierra-Marquez J, Bungert-Plümke S, Franzen A, Mougharbel L, Berrahmoune S, Dassi C, Poulin C, Srour M, Guzman RE, Myers KA. Genotype-phenotype correlation in CLCN4-related developmental and epileptic encephalopathy. Hum. Genet. (2024). https://doi.org/10.1007/s00439-024-02668-z.
He H, Li X., Guzman G.A., Bungert-Plümke S., Franzen A., Lin X., Zhu M., Peng G., Zhang W., Yu Y., Sun S., Huang Z., Zhai Q., Chen Z., Peng j., and Guzman R.E. (2024). Expanding the genetic and phenotypic relevance of CLCN4 variants in neurodevelopmental condition: 13 new patients. J. Neurol. https://doi.org/10.1007/s00415-024-12383-4
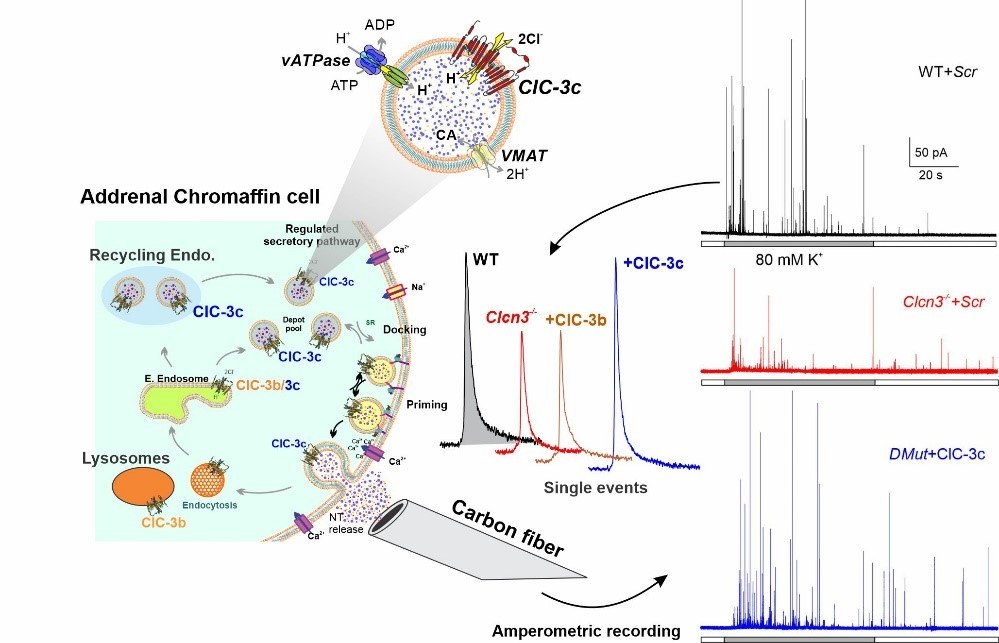
Comini, M., Sierra-Marquez, J., Guzman G.A, Franzen, A., Willuweit, A., Hidalgo, P., Fahlke, C., Guzman, R.E. (2022). CLC anion/proton exchangers regulate secretory vesicle filling and maturation in chromaffin cells. J. Neurosci. 42 (15) 3080-3095; DOI: https://doi.org/10.1523/JNEUROSCI.2439-21.2022. DPG (German Physiological Society) – Paper of the Month Juli 2022
Sierra-Marquez, J., Willuweit, A., Schöneck, M., Bungert-Plümke, S., Gehlen, J., Balduin, C., Müller, F., Lampert, A., Fahlke, C., Guzman, R.E. (2022). ClC-3 regulates the excitability of nociceptive neurons and suppresses inflammatory processes within the spinal sensory pathway. Front. Cell. Neurosci. DOI: 10.3389/fncel.2022.920075.
Guzman, R.E, Sierra-Marquez, J., Bungert-Plümke, Franzen, A., Fahlke, C. (2022). Functional characterization of CLCN4 variants associated with X-linked intellectual disability and epilepsy. Front. Mol. Neurosci. doi.org/10.3389/fnmol.2022.872407
He, H., Guzman, R. E., Cao, D., Sierra-Marquez, J., Yin, F., Fahlke, C., Peng, J., Stauber, T. (2021). The molecular and phenotypic spectrum of CLCN4-related epilepsy. Epilepsia 62, 1401-1415.
Grieschat, M*., Guzman, R. E*., Langschwager, K*., Fahlke, C., Alekov, A. K. (2020). Metabolic energy sensing by mammalian CLC anion/proton exchangers. EMBO Rep 21, e47872. *Equal contribution.