Information Processing in the Retina
Prof. Dr. Frank Müller's Group
Institute for Biological Information Processes
Molecular and Cell Physiology (IBI-1)
Forschungszentrum Jülich
The perception of visual stimuli begins in the retina in our eye. The retina is an outpost of the brain. It contains not only the light-sensitive photoreceptors in the rod and cone varieties, but also an elaborate network of nerve cells that processes the information from the photoreceptors before transmitting it to the brain. In total, there are at least 60 different nerve cell types in the retina. The Müller group investigates how the retinal network is structured and how the different cell types in the retina process and transmit information.
We employ a variety of different methods in our research. We use electrophysiological techniques, such as multi-electrode recordings, methods from biochemistry and molecular biology, as well as immunohistochemistry and confocal laser scanning microscopy. Using imaging techniques, we demonstrate in living nerve cells how the concentration of important intracellular messengers (such as calcium, cAMP, or cGMP) changes during signal processing. We also use optogenetic methods for this purpose.
Analysis of the retinal network
The eyes are our most important sensory organs. They transmit to us 70-80% of the information we learn about our environment. When light falls on a photoreceptor in the retina, a molecular reaction chain is initiated, at the end of which the electrical voltage on the cell membrane changes. This results in less of the messenger substance glutamate being released at the synapses, the connection points to downstream nerve cells, a change that is recognized, processed, and reported on by the downstream cells. Important signal pathways are represented by the bipolar cells. They connect the photoreceptors with the retinal ganglion cells, which then transmit the signals to the brain. There are approximately 1 dozen types of bipolar cells in total. They are not simply collection points for signals that arrive from the photoreceptors, but they filter out important information from the stream of messages, such as news about the environment.

Molecular emergency brake in the eye
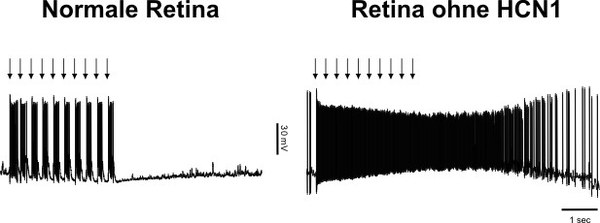
The human eye has remarkable properties. We can see in the night with weak starlight, but also in bright sunshine when ten billion times more light falls into the eye. While the rods are very light-sensitive and enable us to see at night, we see during the day with the less sensitive cones. In a transitional area, e.g. at dusk, both rods and cones are active. The cooperation of rods and cones must then be precisely regulated to maintain the balance between the very different types of photoreceptors. We have elucidated a mechanism that enables this balance at the molecular level.
To do this, rods use the ion channel HCN1. In the twilight range, it reduces the excessive blinding of rods by bright light stimuli. HCN1 is activated when the membrane voltage of the rods changes significantly upon light exposure and then counteracts this change. In doing so, it reduces the extent of the voltage change so that the rod response is not “at the limit”. The strength of the signal that the rods feed into the retinal network is reduced to a reasonable level so as not to disrupt the processing of the cone signals in the retinal network. The ion channel acts like a molecular emergency brake to minimize glare. In mice that can no longer form this ion channel due to a genetic defect, the cells in the retina react as if they were blinded by bright light. By studying how the responses of retinal cells change after the disruption of further signaling proteins and pathways in the rod and cone pathways, we were able to follow the propagation of rod signals throughout the entire retinal network.
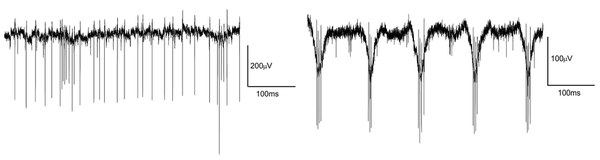
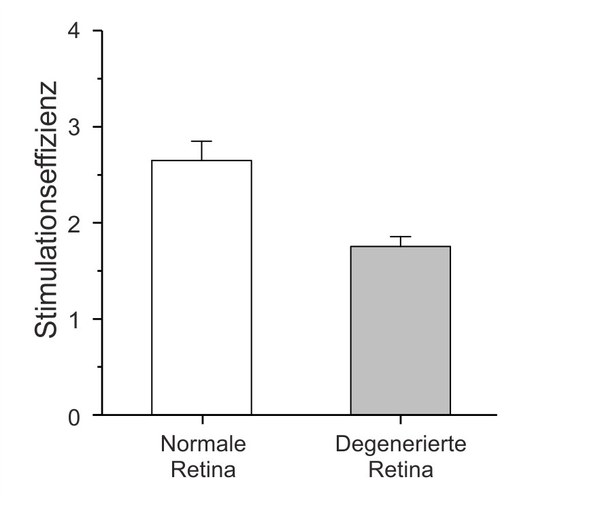
Retinal degeneration and development of new retinal implants
Rods and cones that have died cannot be replaced by the retina. Certain degenerative diseases of the retina lead to extensive loss of photoreceptors and thus blindness. However, the inner network of the retina remains, as does the connection to the brain. One possible therapy is to electrically stimulate the remaining nerve cells in the retina to trigger visual perception. The results achieved with such retinal implants are hopeful, but often fall short of original expectations. Various factors are discussed as reasons for this, including remodeling processes that occur as part of the degeneration process in the retina. We investigate how the anatomical and physiological properties of the retina change as a result of such remodeling processes. For example, we found that the degenerated retina is more difficult to stimulate with implants than the normal retina. This could explain why the performance of current implants often falls short of expectations. Together with colleagues from the Jülich Research Center, the Aachen University Eye Clinic, the RWTH and the University of Duisburg-Essen, we are trying to develop new concepts for electrical stimulation of the retina.
Link to the graduate school “Innovative interfaces to the retina for optimized artificial – InnoRetVision“
Easy-to-understand introduction to the senses
Frings, S. und Müller, F. „Biologie der Sinne – vom Molekül zur Wahrnehmung“. 2. Auflage. Springer Spektrum 2019.
Selected publications
Biswas S, Haselier C, Mataruga A, Thumann G, Walter P, Müller F. Pharmacological analysis of intrinsic neuronal oscillations in rd10 retina. PloS one. 2014;9(6):e99075. doi: 10.1371/journal.pone.0099075. PubMed PMID: 24918437; PubMed Central PMCID: PMC4053359.
Rösch S, Johnen S, Mataruga A, Müller F, Pfarrer C, Walter P. Selective photoreceptor degeneration by intravitreal injection of N-methyl-N-nitrosourea. Investigative ophthalmology & visual science. 2014;55(3):1711-23. doi: 10.1167/iovs.13-13242. PubMed PMID: 24550357.
Rösch S, Johnen S, Müller F, Pfarrer C, Walter P, “Correlations between ERG, OCT, and Anatomical Findings in the rd10 Mouse”, Journal of Ophthalmology. 2014.
Rösch S, Johnen S, Mazinani B, Müller F, Pfarrer C, Walter P. (2015) The effects of iodoacetic acid on the mouse retina. Graefes Arch Clin Exp Ophthalmol; 253(1):25-35.
Rösch S, Aretzweiler C, Müller F, Walter P. (2016) Evaluation of Retinal Function and Morphology of the Pink-Eyed Royal College of Surgeons (RCS) Rat: A Comparative Study of in Vivo and in Vitro Methods. Curr Eye Res, DOI: 10.1080/02713683.2016.1179333.
Rösch S, Werner C, Müller F, Walter P. Photoreceptor degeneration by intravitreal injection of N-methyl-N-nitrosourea (MNU) in rabbits: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2017 Feb;255(2):317-331. doi: 10.1007/s00417-016-3531-7.
Rösch S, Aretzweiler C, Müller F, Walter P. Evaluation of Retinal Function and Morphology of the Pink-Eyed Royal College of Surgeons (RCS) Rat: A Comparative Study of in Vivo and in Vitro Methods. Curr Eye Res. 2017 Feb;42(2):273-281. doi: 10.1080/02713683.2016.1179333
Haselier C, Biswas S, Rösch S, Thumann G, Müller F, Walter P (2017) Correlations between specific patterns of spontaneous activity and stimulation efficiency in degenerated retina. PLoS One 12:e0190048
Rincon Montes V, Gehlen J, Lück S, Mokwa W, Müller F, Walter P, Offenhäusser A (2019) Toward a bidirectional communication between retinal cells an d a prosthetic device – a proof of concept. Front Neurosci 13:367
Meer AV, Berger T, Müller F, Foldenauer AC, Johnen S, Walter P (2020) Establishment and characterization of a unilateral UV-induced photoreceptor degeneration model in the C57Bl/6J mouse. Transl Vis Sci Technol 9:21
Rincon Montes V, Gehlen J, Ingebrandt S, Mokwa W, Walter P, Müller F, Offenhäusser A (2020) Development and in vitro validation of flexible intraretinal probes. Sci Rep 10:19836
Gehlen J, Esser S, Schaffrath K, Johnen S, Walter P, Müller F (2020) Blockade of retinal oscillations by benzodiazipines improves efficiency of electrical stimulation in the mouse model of RP, rd10. Invest Opthalmol Vis Sci 61:37
Halfmann C, Rüland T, Müller F, Jehasse K, Kampa BM (2023) Electrophysiological properties of layer 2/3 pyramidal neurons n the primary visual cortex of a retinitis
