Expertise
The INM provides a comprehensive equipment setup for in-vivo brain imaging. In the Imaging Core Facility (INM-ICF), these devices are consolidated in a structure jointly managed by the INM, and measurement times are allocated to internal and external users through a transparent procedure.
More specific information relating to the system calendars, as well as extra information about subject screening, can be found on the ICF intranet page.
Use & Safety
Use of measurement facilities
The planning, execution and support of individual measurements on the imaging devices is the responsibility of the ICF. Study leaders planning a study firstly must submit an application to the measurement time committee. Without the approval of the measurement time committee, studies cannot be initiated.
The application form (current version 6.3) can be downloaded here: Measurement time application
The application, along with all necessary documents (completed application form, ethical committee opinion, proof of accident insurance coverage), has to be sent to the following email address:
Once the study is approved, the study leaders will contact the ICF to plan the details of the study and coordinate potential dates.
Participant safety
Participants undergoing human measurements in the MRI must not have metallic implants, tattoos in critical areas, etc. The 7T high-field MRI scanner, in particular, carries specific risks and requires careful consideration. Initially, participants themselves declare whether they have potentially MRI-critical surgeries, implants, or tattoos. In the case of potential risks, the INM-4 has developed a "Risk assessment for participants with potential contraindications" (RIS-form) downloadable here: RIS-form
For subjects with significant dental work who will be scanned on the 7 T, an additional form needs to be completed regarding their dental status, which can be found here.
The form(s), along with all relevant documents (e.g., surgical reports, proof of implant materials used, etc.), should be submitted to the following email address:
The ICF staff first assesses whether there are MRI-critical issues with the participants. In cases of uncertainty, other experts are consulted to provide their assessment of the respective cases. If the assessment is negative, the study will not be conducted on those participants. If there are no critical parameters, the participant may proceed.
Participant education is carried out by the study leaders, for which consent forms have been developed. Additionally, some studies may have additional requirements from ethics committees to be considered.
Radiation Protection
The ICF radiation protection team is responsible for radiation protection across all institutes and works closely with the radiation protection team at INM-5. Both teams are responsible for protecting employees and the environment from potentially harmful effects of ionizing radiation or radioactive substances.
The ICF radiation protection team's area of activity extends to the control areas in buildings 15.2 and 15.16 (ground floor, first floor) and includes a variety of general and specific tasks, such as carrying out measurement routines and contamination checks as well as monitoring the dose of employees. Other important tasks include instruction and training in the safe handling of radioactive substances, supporting external companies and monitoring compliance with all applicable rules. The radiation protection team is also responsible for preparing documentation and approval documents as well as accompanying recurring tests.
Contact persons:
Ingo Spahn (B-SSB, INM-5) Tel: 4450
Armin Kieven (ICF) Tel: 4770
Lisa Fittkow (ICF) Tel: 85893
Jan Weiss (ICF) Tel: 4770
Equipment
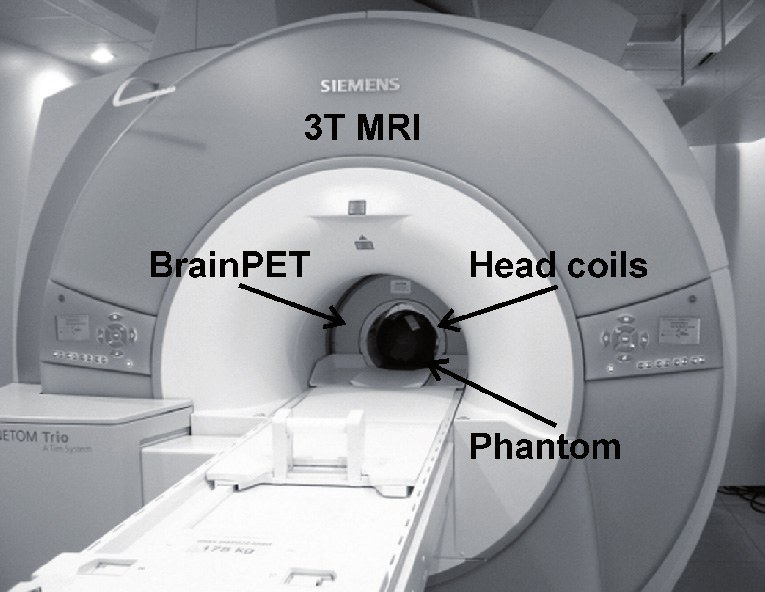
Data Management
Sensitive personal data is handled throughout the entire scope of the ICF. This starts with the collection of participants' personal data and the assignment of a patient ID by the INM administration.
The raw data from the imaging devices is securely stored in a non-manipulatable manner. The same applies to the image files derived from the raw data. Both types of data can be used for further scientific studies or processing. Depending on the study, the data may also be used for medical diagnostics. The ICF runs its own data servers, to which all scanners are connected. Internal users are granted access exclusively to their own data and can access it at any time, while external users do not have direct access to the data server. External requests are routed through an intermediary server and then directed to the ICF Storage data server.
Access to the servers is granted by the ICF, pleas contact Mr. Gruijters, t.gruijters@fz-juelich.de.
