Die Zukunft der Behandlung menschlicher Erkrankungen
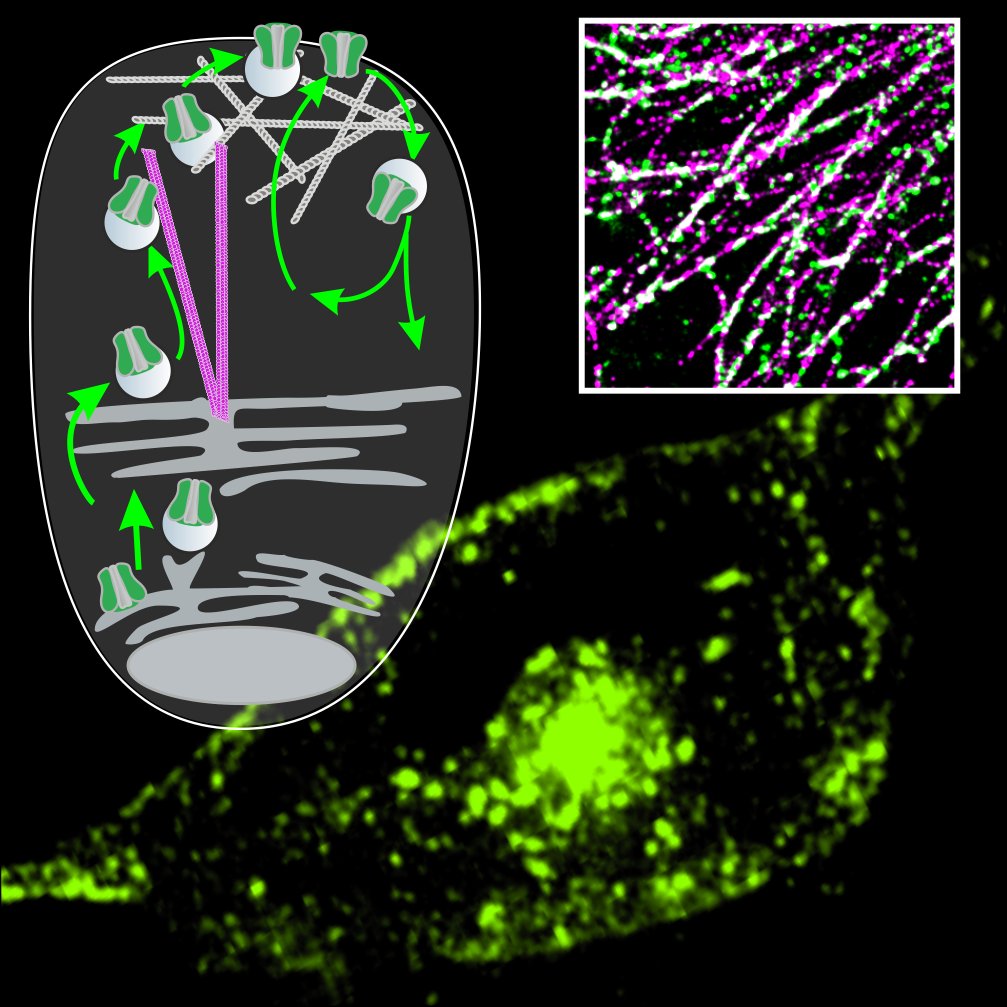
Ionenkanäle, Ionentransporter und Rezeptoren erlauben Zellen, auf extrazelluläre Signale zu reagieren und mit anderen Zellen zu kommunizieren. Wir benutzen eine Kombination aus physikalischen, biologischen, mikroskopischen und computer-gestützten Verfahren, um die molekularen Mechanismen und zellulären Funktionen dieser Proteine zu untersuchen. Unser Ziel ist, die Rolle dieser Proteine unter pathologischen Bedingungen zu verstehen und Strategien zur Korrektur von Fehlfunktionen zu entwickeln, die in Zukunft zur Behandlung menschlicher Erkrankungen eingesetzt werden können. Mehr über uns...
Direktor: Prof. C. Fahlke
Forschungsthemen
Meldungen und Termine
Loading
Loading