Spin-Selective Electron Transport Through Single Chiral Molecules
Mohammad Reza Safari, Frank Matthes, Claus M. Schneider, Karl-Heinz Ernst and Daniel E. Bürgler
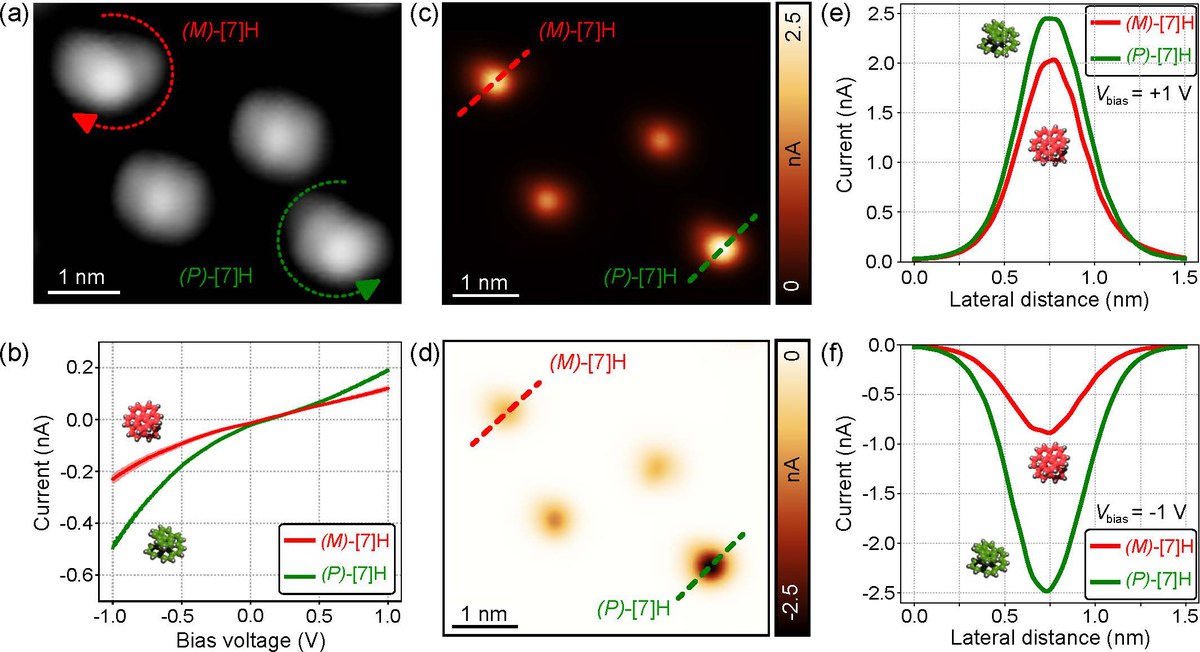
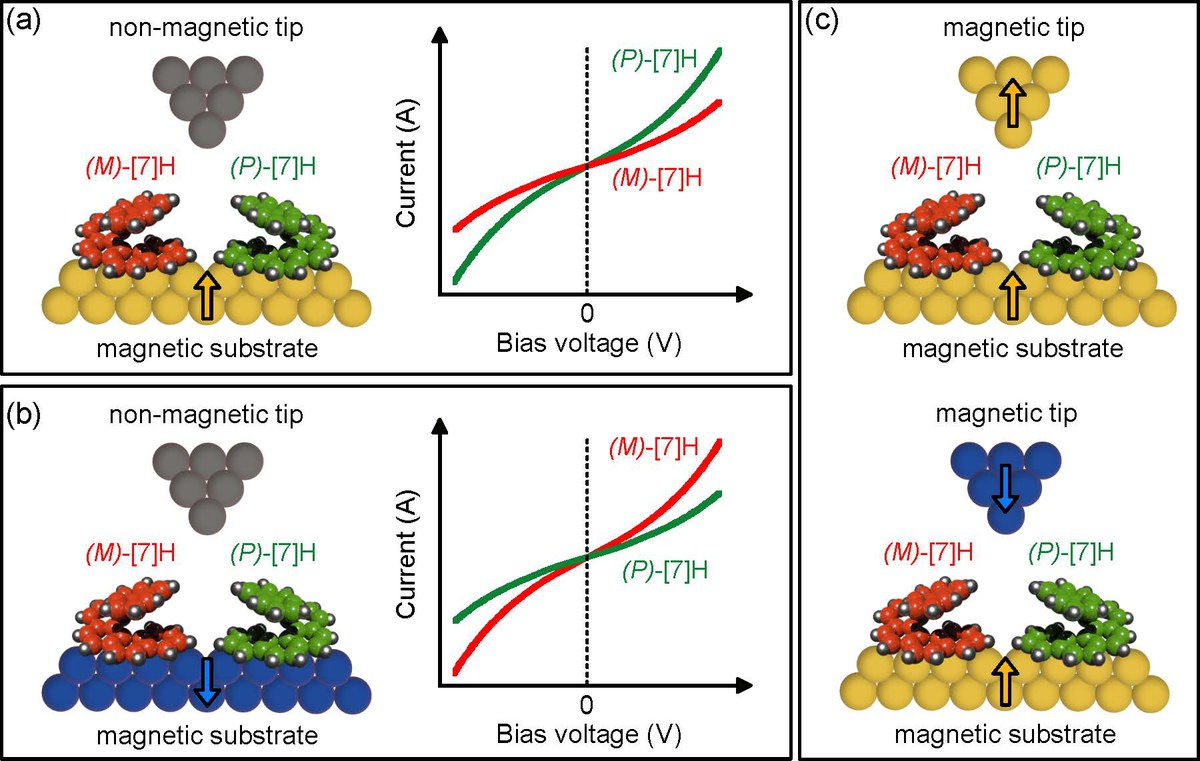
The interplay between chirality and magnetism is a source of fascination among scientists for over a century. In recent years, chirality-induced spin selectivity (CISS) has attracted renewed interest. It is observed that electron transport through layers of homochiral molecules leads to a significant spin polarization of several tens of percent. Despite the abundant experimental evidence gathered through mesoscopic transport measurements, the exact mechanism behind CISS remains elusive. This study reports spin-selective electron transport through single helical aromatic hydrocarbons that are sublimed in vacuo onto ferromagnetic cobalt surfaces and examined with spin-polarized scanning tunneling microscopy (SP-STM) at a temperature of 5 K. Direct comparison of two enantiomers under otherwise identical conditions revealed magnetochiral conductance asymmetries of up to 50% when either the molecular handedness is exchanged or the magnetization direction of the STM tip or Co substrate is reversed. Importantly, the results rule out electron–phonon coupling and ensemble effects as primary mechanisms responsible for CISS.
Evaluation of magnetochiral electron tunneling asymmetries with SP-STM
CISS for a single pair of [7]H enantiomers