Activity and selectivity of ethanol electrooxidation by octahedral PtNiRh nanoparticles
June 2017
by Nina Erini, Vera Beermann, Martin Gocyla, Manuel Gliech, Marc Heggen, Rafal E Dunin-Borkowski, and Peter Strasser
Direct ethanol fuel cells are attractive power sources based on a biorenewable, high energy-density fuel. Their efficiency is limited by the lack of active anode materials which catalyse the breaking of the C−C bond coupled to the 12-electron oxidation to CO2.
We report shape-controlled PtNiRh octahedral ethanol oxidation electrocatalysts with excellent activity and previously unachieved low onset potentials, while being highly selective to complete oxidation to CO2.
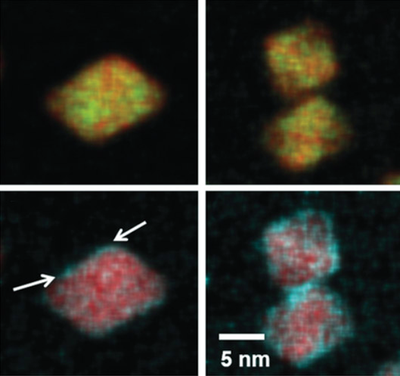
A quantitative STEM/EDX analysis shows an inhomogeneous distribution of Pt and Ni within the octahedral nanoparticle catalysts and a speckled distribution of Rh over the nanoparticle surface, which gives rise to isolated Rh-rich patches. Our comprehensive characterisation and in situ electrochemical ATR studies suggest that the formation of a ternary surface site ensemble around the octahedral Pt3Ni1Rhx nanoparticles plays a crucial mechanistic role for this behaviour.
Further reading:
Nina Erini, Vera Beermann, Martin Gocyla, Manuel Gliech, Marc Heggen, Rafal E Dunin-Borkowski, and Peter Strasser:
The effect of surface site ensembles on the activity and selectivity of ethanol electrooxidation by octahedral PtNiRh nanoparticles,
Angewandte Chemie 129 (2017) 6633-6638.
