Nickel phosphide catalysts for water oxidation
July 2017
by Junyuan Xu, Xian-Kui Wei, José Diogo Costa, José Luis Lado, Bryan Owens-Baird, Liliana P L Gonçalves, Soraia P S Fernandes, Marc Heggen, Dmitri Y Petrovykh, Rafal E Dunin-Borkowski, Kirill Kovnir, and Yury V. Kolen’ko
An approach to significantly enhance the performance of the cost-effective nickel phosphide catalyst for electrochemical water oxidation has been developed via interfacing with Mg oxide-hydroxide. We have synthesized Ni2P nanoparticles anchored on Mg2O(OH)2-like phase supported on carbon paper. During the oxygen evolution reaction, the well-defined Ni2P nanoparticles serve as precursors for the immediate formation of active and stable nanostructured nickel hydroxide catalyst.
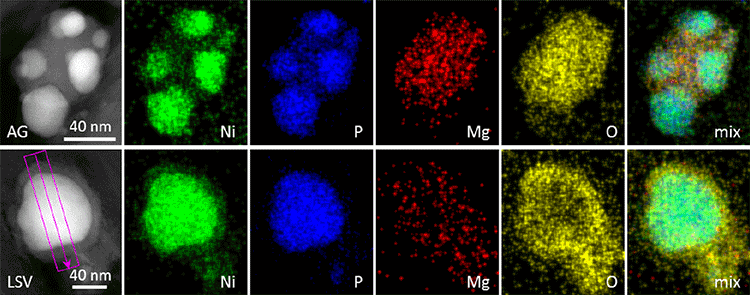
As the anode for the oxygen evolution reaction in an alkaline electrolyte, the electrode shows a modest Tafel slope of 48 mV dec–1 and a large turnover frequency of 0.05 s–1 at an overpotential of 0.4 V. Microstructure and composition studies of the catalyst suggest that interfacial strain between Mg- and Ni-containing phases is responsible for high catalytic activity. A significant increase in catalytic activity upon the combination of magnesium compound and transition-metal phosphide suggests an interesting strategy for the controlled and reproducible preparation of active Earth-abundant oxygen-evolving catalysts.
Further reading:
Junyuan Xu, Xian-Kui Wei, José Diogo Costa, José Luis Lado, Bryan Owens-Baird, Liliana P L Gonçalves, Soraia P S Fernandes, Marc Heggen, Dmitri Y Petrovykh, Rafal E Dunin-Borkowski, Kirill Kovnir, and Yury V. Kolen’ko:
Interface engineering in nanostructured nickel phosphide catalyst for efficient and stable water oxidation,
ACS Catalysis 7 (2017) 5450–5455.
