New strategy for breaking the cellular membrane barriers
Efficient incorporation of biomolecules e.g. proteins, mRNA, or anti-cancer therapeutics into living cells is a major pharmacological challenge. There are several cellular uptake routes involved in nanoparticle incorporation. Of the possible delivery routes, we selected the most efficient and fastest, complexation with lipid nanoparticles that fuse directly with biomembranes, for numerous cargo deliveries into living cells.
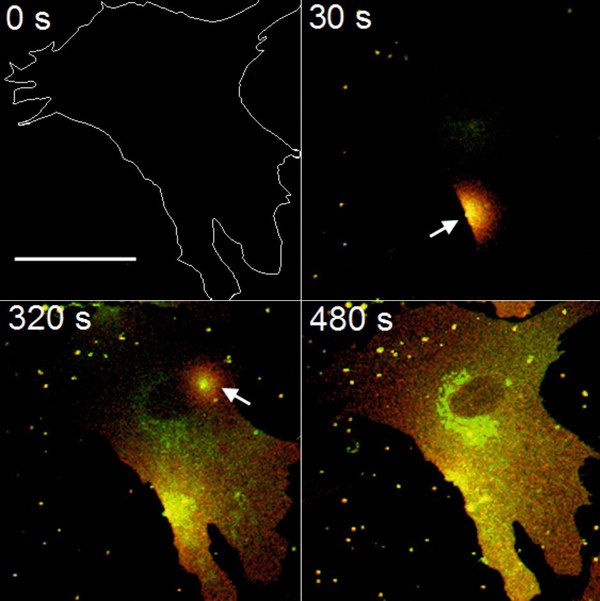
Delivery of biomolecules to cells is a crucial step in all pharmacological treatments and in most life sciences experiments. Lipid vesicles, called liposomes, are widely used for this purpose based on their high bioavailability and low toxicity. The main drawback of such liposomes is that their cellular uptake shows a strong preference of endocytosis. A faster and more effective alternative for efficient molecular delivery is the induction of membrane fusion between liposomes and mammalian cells whereby the cellular plasma membrane is merged with the liposomal membrane, and the cellular cytoplasm with the liposomal lumen. Therefore, such liposomes are highly appropriate for simultaneous and multiple live cell functionalizations. Moreover, molecular delivery by fusion completely bypasses endosomes, therefore avoiding immediate degradation of the cargo. For this reason, our main goal was to achieve a highly fusogenic character for liposomal nanocapsules, and to use those particles as universal carriers for biomolecules in pharmacological and biomedical applications.
Fusogenic liposomes have successfully been developed in IBI-2. They consist exclusively of lipids and an aromatic membrane component thus avoiding immune reactions. Due to the fluorescence character of aromatic molecules, intracellular liposomal imaging became feasible. Using such liposomes as molecular carriers, we efficiently incorporated lipids (link to movie), proteins (link to movie), and mRNA (link to movie) into mammalian cells. Additionally, new pharmacological strategies have been developed for the activation of immune cells against cancer cells and improving cancer therapy efficiency. Our actual study in cooperation with Reynolds Oklahoma Center on Aging (ROCA, Oklahoma City, USA) focuses on the efficient incorporation of polyphenols against oxygen radicals into the cerebral microvascular endothelia of rodents. Especially aged organisms benefit from the anti-oxidative effect of those drugs as shown by their significantly improved neurovascular coupling responses (link to topic: Molecular delivery to cerebral microvasculature). Moreover, we also analyze the interaction of those lipid nanocapsules with the cargo itself (e.g. mRNA), as well as with the surrounding body fluid (blood serum) or basal membranes.
Fusion vs. Endocythosis


Sphingomyeline (green) delivery by fusogenic liposomes (red)

The actin-specific GFP-LifeAct peptide (green) delivered by fusogenic liposomes (red)

Selected publications:
1. Kolasinac, Bier, Schmitt, Yabluchanskiy, Neumeier, Merkel, and Csiszár (2021) Delivery of the radionuclide 131I using cationic fusogenic liposomes as nanocarriers. Int J Mol Sci. 22 (1): 457
2. Wiedenhoeft, Braun, Springer, Teske, Noetzel, Merkel, and Csiszár (2020) The Basement Membrane in a 3D Breast Acini Model Modulates Delivery and Anti-Proliferative Effects of Liposomal Anthracyclines. Pharmaceuticals 13(9), 256
3. Kleusch, Monzel, Sridhar, Hoffmann, Csiszár, and Merkel (2020) Fluorescence correlation spectroscopy reveals interaction of some microdomain-associated lipids with cellular focal adhesion sites. Int J Mol Sci. 21 (21) 8149
4. Wiedenhoeft, Tarantini, Nyúl-Tóth, Yabluchanskiy, Csipo, Balasubramanian, Lipecz, Kiss, Csiszár, Csiszar, Ungvari (2019) Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience, 41(6): 711
5. Hoffmann, Hersch, Gerlach, Dreissen, Springer, Merkel, Csiszar, Hoffmann (2020) Complex Size and Surface Charge Determine Nucleic Acid Transfer by Fusogenic Liposomes, Int J Mol Sci. 21(6): 2244
6. Hoffmann, Hersch, Merkel, Csiszar, Hoffmann (2019) Changing the way of entrance: highly efficient transfer of mRNA and siRNA via fusogenic nano-carriers, J of Biomed Nanotech. 15: 1-14
7. Kolasinac, Jaksch, Dreissen, Braeutigam, Merkel, and Csiszár (2019) Influence of Environmental Conditions on the Fusion of Cationic Liposomes with Living Mammalian Cells, Nanomaterials, 9 (7): 1025
8. Kolasinac, Kleusch, Braun, Merkel, and Csiszár (2018) Deciphering the Functional Composition of Fusogenic Liposomes, Int J Mol Sci. 19: 346
9. Kube, Hersch, Naumovska, Gensch, Hendriks, Franzen, Landvogt, Siebrasse, Kubitscheck, Hoffmann, Merkel, Csiszár (2017) Fusogenic Liposomes as Nanocarriers for Delivery of Intracellular Proteins, Langmuir 33: 1051
10. Braun, Kleusch, Naumovska, Merkel, and Csiszár (2016) A Bioanalytical Assay to Distinguish Cellular Uptake Routes for Liposomes, Cytometry A 89A: 301-308
11. Hersch, Wolters, Ungvari, Gautam, Deshpande, Merkel, Csiszar, Hoffmann, Csiszár (2016) Biotin-conjugated fusogenic liposomes for high-quality cell purification, J Biomater Appl. 6: 846
12. Csiszár, Csiszar, Pinto, Gautam, Kleusch, Hoffmann, Tucsek, Toth, Sonntag, Ungvari (2015) Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rates, J Gerontol A 70:303
13. Naumovska, Ludwanowski, Hersch, Braun, Merkel, Hoffmann, Csiszár (2014) Plasma membrane functionalization using highly fusogenic immune activator liposomes, Acta Biomater. 10: 1403
14. Kleusch, Hersch, Hoffmann, Merkel, Csiszár (2012) Fluorescent lipids: functional parts of fusogenic liposomes and tools for cell membrane labeling and visualization, Molecules. 17: 1055
15. Csiszár, Hersch, Dieluweit, Biehl, Merkel, and Hoffmann (2010) Novel Fusogenic Liposomes for Fluorescent Cell Labeling and Membrane Modification, Bioconjugate Chemistry 21: 537-543
Book chapter
1. Liposomes in Analytical Methodology, Pan Stanford Publishing (2016) edited by Katie A. Edwards: Chapter11 Fluorescent Dye-Encapsulating Liposomes for Cellular Visualization by Agnes Csiszár and Rudolf Merkel p: 417-457.
Patents and patent pendings
1. Hoffmann, Csiszár, Hersch, Merkel: Mischung amphipathischer Moleküle und Verfahren zur Zellmembranmodifikation durch Fusion, 2015
Nr.: JP 2016504486
2. Hoffmann, Csiszár, Hersch, Merkel, Hoffmann, Zantl: Method for producing a fusion mixture for transferring a charged molecule into and/or through a lipid membrane
Nr.: EP 3115039 A1
3. Hoffmann, Csiszár, Hersch, Merkel, Hoffmann, Zantl: Method for producing a fusion mixture for transferring a charged molecule into and/or through a lipid membrane
Nr.: US 20170009255 A1
4. Csiszár, Hoffmann, Braun: Verfahren zum Einbau von lysosomalen- Transmembranproteinen in zelluläre Membranen, 2015
Nr.: DE 102013005154 A1
5. Csiszár, Kleusch, Hoffmann, Merkel: Molecule mixture comprising an amphipathic molecule type a, which has a positive total charge in the hydrophilic range, and an amphipathic molecule type b and a polyphenol c, method for producing said molecule mixture and use thereof, 2017
Nr.: US 20150037398 A1
contact:
Dr. Agnes Csiszar
Senior Scientist
- Institute of Biological Information Processing (IBI)
- Mechanobiology (IBI-2)
Room R 2005

