Deciphering mechanical signal processing at the cell-basement membrane-matrix interface between cells, basement membrane, and extracellular matrix
All cells in every tissue experience dynamic mechanical stresses and are part of their physiological niche. We study mechanical ECM cues such as stiffness and shear strain as fundamental mechanobiological modulators of healthy and diseased cell function. Cells interact with the surrounding extracellular matrix (ECM) across a basement membrane (BM) barrier. A functional cell-BM-ECM interface is essential part of almost all tissues in the human body from epithelia to brain and orchestrates normal tissue development and function. A malfunctioned interplay of cells and the ECM is linked with severe diseases, such as cancer.
Our work aims at the understanding of mechanobiological signaling cascades that regulate tissue function. In order to recapitulate reciprocal signal processing between cells, the BM and ECM, we apply physiological mechanical ECM cues to biomimetic 3D cell cultures of BM-covered breast spheroids. We use these versatile cell models to study fundamental signaling circuits of cellular mechanoadaptation that orchestrate epithelial tissue development and neuronal network function.

The Basement Membrane as cell invasion barrier
We focus on the BM’s role as a physical cell migration barrier and signaling platform for reciprocal mechanotransduction ECM stresses. Cells sense ECM stiffness by highly dynamic BM-penetrating protrusions that modulate actomyosin-driven cell contraction and protrusive forces. We investigate the underlying signal processing circuits of mechanical BM disruption and loss of tissue function.
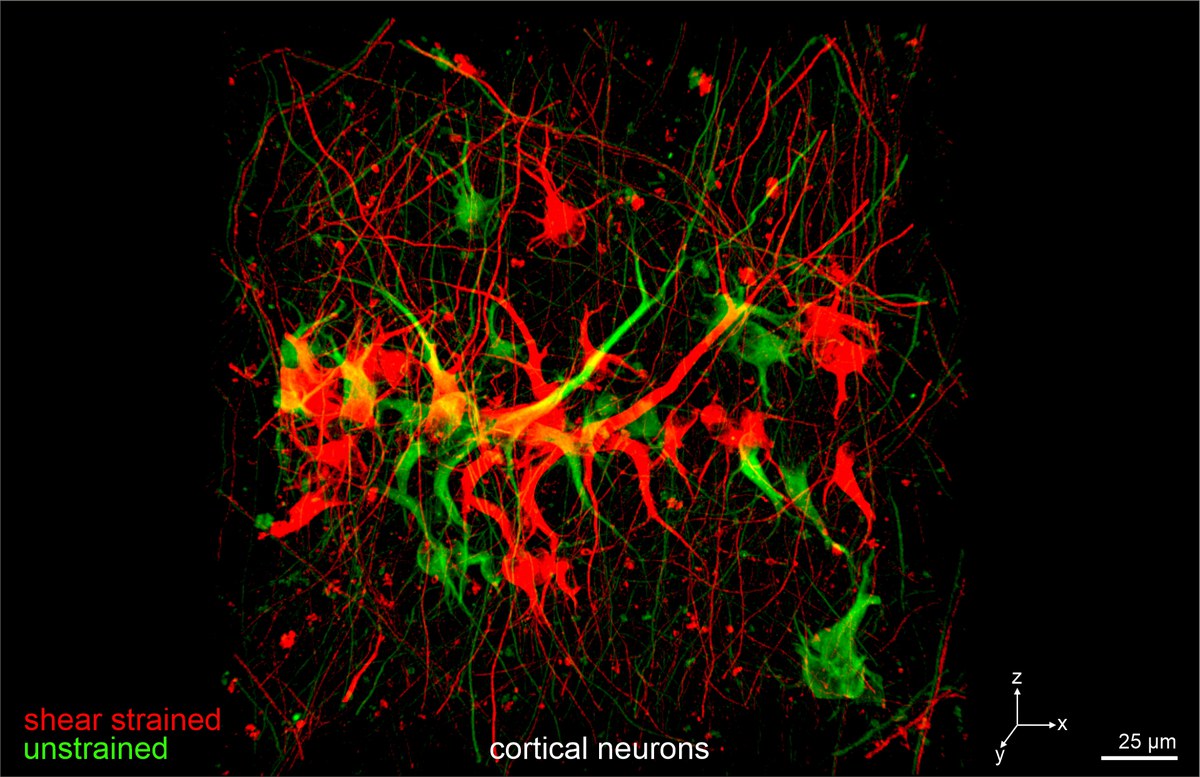
ECM-transmitted shear strain regulates tissue development
ECM-transmitted shear strain is a fundamental but poorly understood mechanical cue acting on cell differentiation and homeostasis. We develop and apply unique cell culture devices to apply nature-like ECM-transmitted shear strain on developing epithelial breast gland spheroids and neuronal networks. These unique approaches enable to study cellular mechanoadaptation mechanisms in response to nature-like ECM shear strain and stiffness.
Selected publications
1. Esser, L., Springer, R., Dreissen, G., Lövenich, L., Konrad, J., Hampe, N., Merkel, R., Hoffmann, B. & Noetzel, E. Elastomeric Pillar Cages Modulate Actomyosin Contractility of Epithelial Microtissues by Substrate Stiffness and Topography. Cells 12, 1256 (2023). https://www.mdpi.com/2073-4409/12/9/1256
2. Friedland, F., Babu, S., Springer, R., Konrad, J., Herfs, Y., Gerlach, S., Gehlen, J., Krause, H.J., De Laporte, L., Merkel, R. & Noetzel, E. ECM-transmitted shear stress induces apoptotic cell extrusion in early breast gland development. Front Cell Dev Biol 10, 947430 (2022). 10.3389/fcell.2022.947430
3. Eschenbruch, J., Dreissen, G., Springer, R., Konrad, J., Merkel, R., Hoffmann, B. & Noetzel, E. From Microspikes to Stress Fibers: Actin Remodeling in Breast Acini Drives Myosin II-Mediated Basement Membrane Invasion. Cells 10 (2021). 10.3390/cells10081979
4. Gaiko-Shcherbak, A., Eschenbruch, J., Kronenberg, N.M., Teske, M., Wolters, B., Springer, R., Gather, M.C., Merkel, R., Hoffmann, B. & Noetzel, E. Cell Force-Driven Basement Membrane Disruption Fuels EGF- and Stiffness-Induced Invasive Cell Dissemination from Benign Breast Gland Acini. Int J Mol Sci 22 (2021). 10.3390/ijms22083962
5. Wiedenhoeft, T., Braun, T., Springer, R., Teske, M., Noetzel, E., Merkel, R. & Csiszar, A. The Basement Membrane in a 3D Breast Acini Model Modulates Delivery and Anti-Proliferative Effects of Liposomal Anthracyclines. Pharmaceuticals (Basel) 13 (2020). 10.3390/ph13090256
6. Fabris, G., Lucantonio, A., Hampe, N., Noetzel, E., Hoffmann, B., DeSimone, A. & Merkel, R. Nanoscale Topography and Poroelastic Properties of Model Tissue Breast Gland Basement Membranes. Biophys J 115, 1770-1782 (2018). 10.1016/j.bpj.2018.09.020
7. Gaiko-Shcherbak, A., Fabris, G., Dreissen, G., Merkel, R., Hoffmann, B. & Noetzel, E. The Acinar Cage: Basement Membranes Determine Molecule Exchange and Mechanical Stability of Human Breast Cell Acini. PLoS One 10, e0145174 (2015). 10.1371/journal.pone.0145174
Contact:
Dr. Erik Noetzel-Reiss
Senior Scientist and Group Leader
- Institute of Biological Information Processing (IBI)
- Mechanobiology (IBI-2)
Room 2006

