Evaluation of Alternative Lithium Salts for High-voltage Lithium-ion Batteries
Lithium Plating Morphology Identified as Relevant Factor
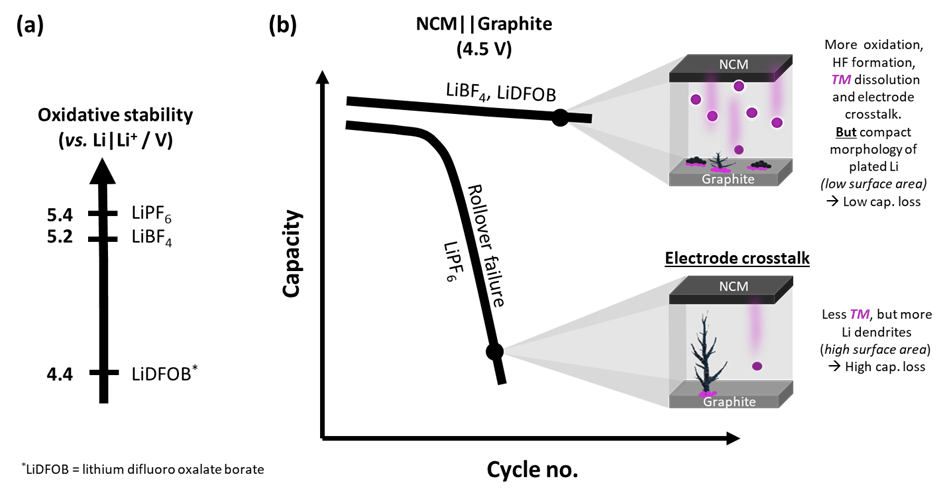
17 December 2024 – One of the main origins of capacity fade in high-energy lithium-ion batteries (LIB) is 'electrode crosstalk'. Transition metals dissolve from the cathode and deposit on the anode. Lithium salts, which are the main component of the electrolyte, can influence this process and improve capacity retention of the battery cells. In a recent study, a team from MEET Battery Research Center at the University of Münster and from Helmholtz Institute Münster of Forschungszentrum Jülich has investigated the mechanistical influence of various lithium salts on the degradation behavior of high-energy LIB.
Behavior of Lithium Salts Counterintuitive at First Glance
The researchers compared six different lithium salts with each other: among them are the state-of-the-art (SOTA) lithium hexafluorophosphate, lithium tetrafluoroborate and lithium difluoro(oxalate)borate. They identified that the latter two salts retain the capacity of the cells better than SOTAsalt. At the same time, these salts decompose more and thus lead to increased 'electrode crosstalk'. “This behavior, counterintuitive at first glance, can be explained by differences in the lithium plating morphology caused by different salts in the electrolyte,” says Anindityo Arifiadi, PhD student at MEET Battery Research Center and the International Graduate School BACCARA (Battery Chemistry, Characterization, Analysis, Recycling and Application). While electrolyte with the SOTA salt leads to lithium plating with dendritic morphology in the anode, i.e., high surface area, the structure is more compact when lithium tetrafluoroborate or lithium difluoro(oxalate)borate is employed. As a result, parasitic side reactions of lithium and lithium losses decrease, consequently increases the capacity retention. “The lithium plating morphology therefore plays a more important role in affecting capacity retention in high-voltage LIBs than the amount of ‘electrode crosstalk’,” Arifiadi classifies the results.
However, the practical use of lithium tetrafluoroborate and lithium difluoro(oxalate)borate can be challenging, as more gas are formed during cycling compared to the case of the SOTA salt. Dr Johannes Kasnatscheew, Head of the Research Division Materials at MEET Battery Research Center, explains: “The effect of lithium salts must be explored further. Only if we understand it in detail, we can design tailor-made electrolytes for high-voltage LIB. The learnings from this study can serve as a guidance.”
Entire Study Available
The detailed results have been published by the authors Anindityo Arifiadi, Tobias Brake, Christian Lechtenfeld, Julius Buchmann, Feleke Demelash, Dr Simon Wiemers-Meyer and Dr Johannes Kasnatscheew, MEET Battery Research Center, Lennart Wichmann, Peng Yan, Dr Gunther Brunklaus and Dr Isidora Cekic-Laskovic, Helmholtz Institute Münster as well as Prof. Dr Martin Winter, MEET Battery Research Center and Helmholtz Institute Münster, in the journal “Small”.
