Interfacial Spectroelectrochemistry

Overview
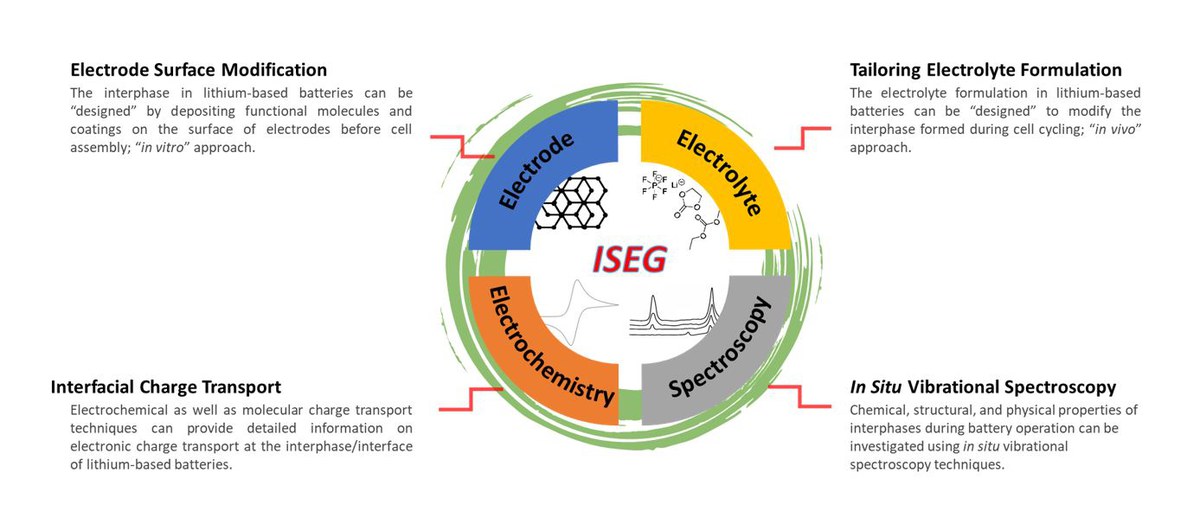
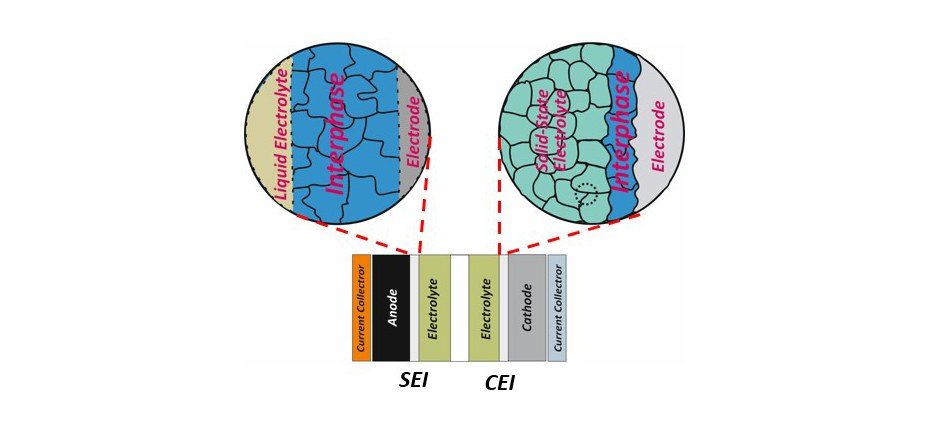
A transition from conventional to next-generation rechargeable lithium batteries includes not only the development and modification of electrode structures and electrolyte formulations but also the boundary of the electrode and electrolyte, the interface, whereby the reaction of electrodes and electrolytes, so-called "interphases" are formed. Instability of convential electrolytes against anode and cathode at high operating voltages leads to formation of a solid electrolyte interphase (SEI) and a cathode electrolyte interphase (CEI) at the expense of active lithium and electrolyte materials.
The Young Investigator Group "Interfacial Spectroelectrochemistry Group" (ISEG) of Helmholtz Institute Münster (HI MS) focuses on designing suitable interphases by taking in vivo and in vitro approaches to improve the chemical, physical, and mechanical properties of SEI and CEI. To better understand the surface phenomena on a molecular level in rechargeable lithium batteries, in situ near-field vibrational spectroscopy techniques are utilized.
Tailoring Electrolyte Formulation
A robust SEI or CEI can significantly influence a rechargeable lithium battery's performance, cyclability, and safety by preventing continuous electrolyte decomposition. Depending on the operation conditions, conventional carbonate-based electrolytes fail to a large extent to form an optimal interphase in case of electrode materials of next-generation rechargeable lithium batteries, like Si-based anode and Ni-rich cathode materials.
Toward reducing the effect of side reactions and tuning the properties of interphases, the development and incorporation of molecular electrolyte additives is considered an essential strategy. Within ISEG, HI MS develops new electrolyte formulations by adding small amounts of molecular additives as an effective approach to improve the interphase properties in the conventional and next-generation high voltage battery systems.
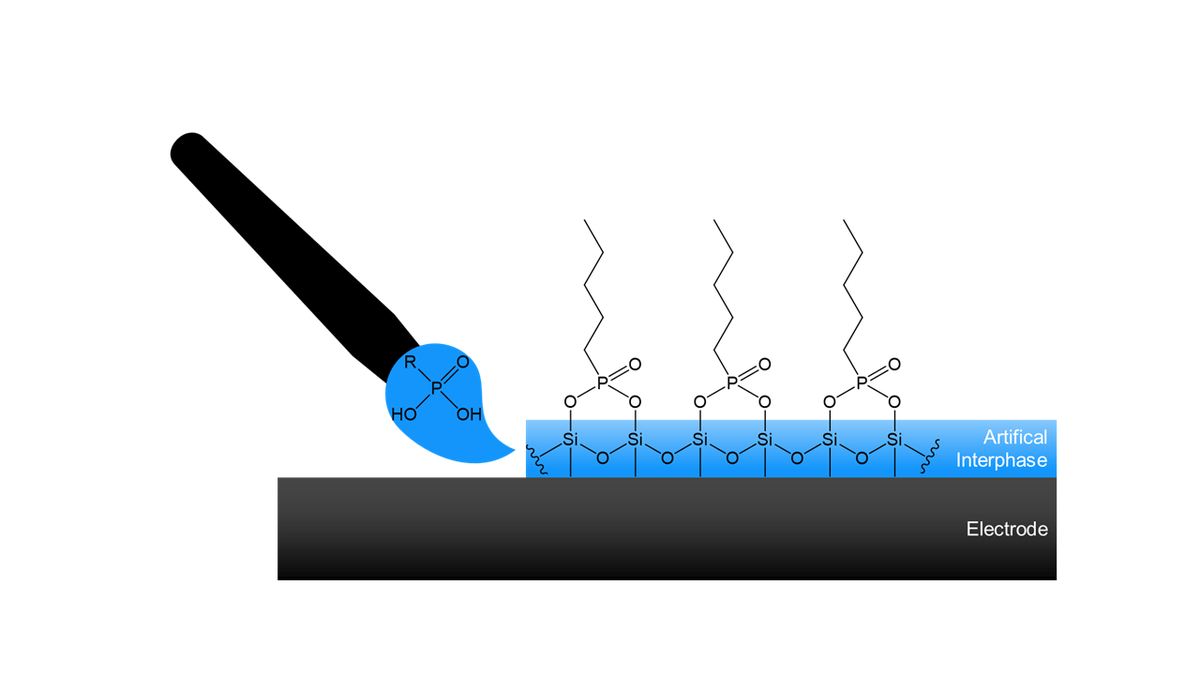
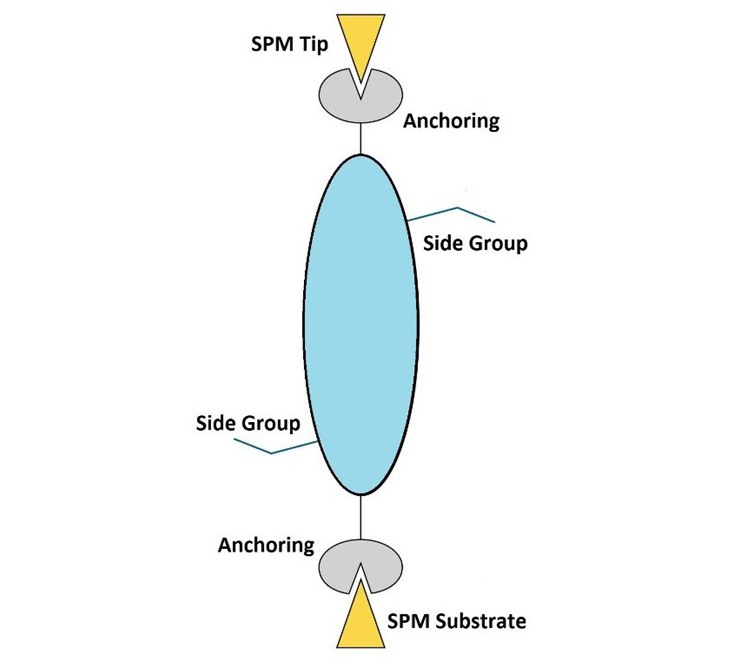
Electrode Surface Modification
A stable interphase can be artificially designed by treating the electrode surfaces with organic and inorganic materials before the cell assembly (in vitro approach). The artificial interphase design offers more flexibility to address and tune specific properties, such as chemical composition, transport, stability, and elasticity. In ISEG, molecular systems with proper anchoring sides are used to form a tailored layer, which functions as an artificial SEI or CEI. Fundamental molecular charge transport and electrochemical techniques are employed to preselect the best molecular systems and monitor their functionality as artificial interphases (see section "Charge Transport through interphases").
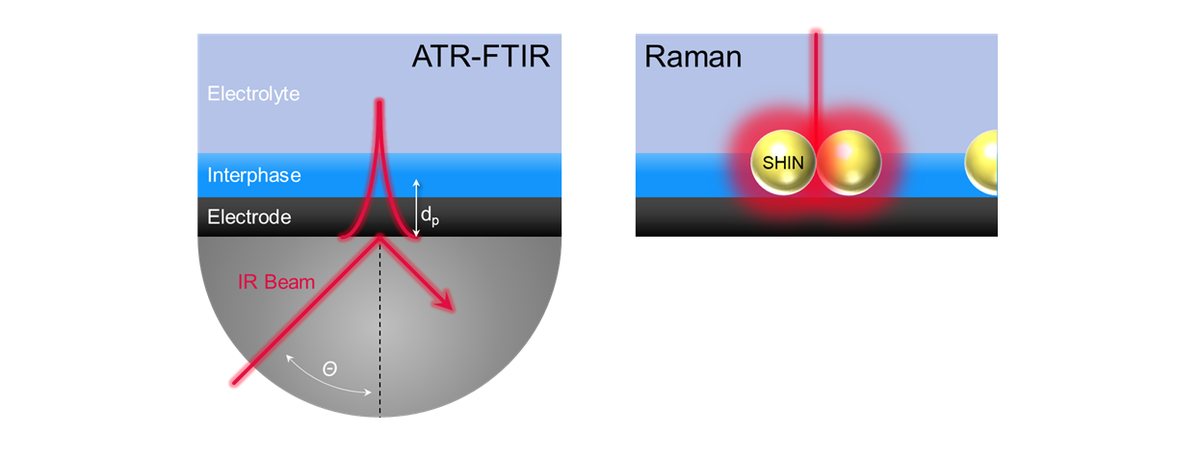
In Situ Vibrational Spectroscopy
An accurate understanding and model description of the interphase processes are unavailable despite intensive scientific efforts. This is partly due to the challenging aspects of developing in situ / operando techniques to establish a real-time mechanistic understanding of the formation, degradation, and aging of the interphases in rechargeable lithium batteries.
In situ / operando vibrational spectroscopy techniques can be utilized to detect unknown molecular species of the components constituting the interphases and identify the respective characteristics of surface chemistries. ISEG is committed to developing advanced in situ / operando Raman and IR spectroscopy techniques to study processes occurring in the interphase.
Charge Transport through Interphases
Electron tunneling or hopping through the interphase causes electrolyte component degradation and leads to continuous interphase growth. Therefore, by observing the electronic charge transfer of single molecular systems, the feasibility of different molecular systems can be estimated as one of the key factors to define a battery's electrochemical performance.
In collaboration with other groups, ISEG employs molecular charge transport techniques combined with electrochemistry to correlate the battery's overall performance with the fundamental electron transfer properties in the interphase.
Selected Publications:
Advanced Energy Materials 2024, 2402187, DOI: 10.1002/aenm.202402187
Journal of Physical Chemistry C 2024, 128, 16, 6868-6876, DOI: 10.1021/acs.jpcc.4c00232
Advanced Energy Materials 2023, 2303568, DOI: 10.1002/aenm.202303568
Small 2023, 2302486, DOI: 10.1002/smll.202302486
Small 2023, 2300425, DOI: 10.1002/sstr.202300425
Advanced Energy Materials 2023, 2300827, DOI: 10.1002/aenm.202300827
Journal of Inorganic and Organometallic Polymers and Materials 2023, 1574-1451, DOI: 10.1007/s10904-023-02693-x
ACS Applied Materials & Interfaces 2023, 5, 6676–6686, DOI: 10.1021/acsami.2c17958
Journal of Power Sources 2023, 557, 232570, DOI: 10.1016/j.jpowsour.2022.232570
Advanced Energy Materials 2022, 202504, DOI: 10.1002/aenm.202202504
Electrochemistry Communications 2022, 140, 107332, DOI: 10.1016/j.elecom.2022.107332
Journal of Power Sources 2022, 549, 232118, DOI: 10.1016/j.jpowsour.2022.232118
Contact
Dr. Baghernejad Masoud
Young Investigator Group Leader Nachwuchsgruppenleiter
- imd
- imd-4
Room 1

