High Transfer Rates in Lithium Metal Batteries Analysed
High lithium transfer numbers of polycarbonate-based polymer electrolytes reduce dendrite formation and provide increased lithium metal battery life.
12 April 2023 – Compared to conventional liquid electrolytes, polycarbonate-based solid polymer electrolytes in lithium metal batteries have a higher lithium transfer number with less dendrite formation and thus a longer lifetime. In their new study, scientists from Helmholtz Institute Münster (HI MS; IEK-12) of Forschungszentrum Jülich, the Institute of Physical Chemistry of the University of Münster and the MEET Battery Research Center of the University of Münster analyse the reasons for this.
High Lithium Transfer Rates in Lithium Metal
The material lithium metal represents a hope for achieving a high energy density of battery cells, for example in use in mobile applications. For a long-lasting lithium metal battery, a high lithium transfer number is necessary to suppress the premature formation of lithium dendrites and the associated short-circuiting of the cell.
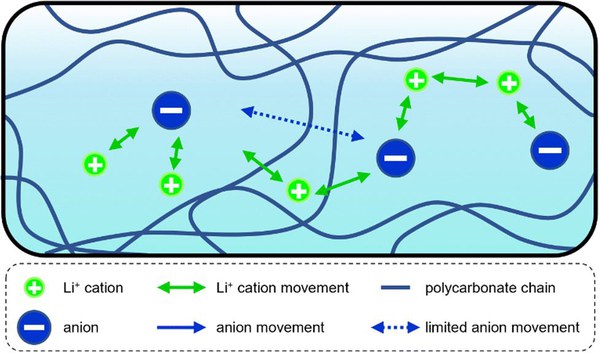
In their collaborative project, the team around Dr Mariano Grünebaum from the Helmholtz Institute Münster and Prof. Dr Andreas Heuer from Helmholtz Institute Münster and the Institute of Physical Chemistry at the University of Münster investigated the central question of why polycarbonate-based polymer electrolytes, specifically polyethylene carbonate (PEC) and polypropylene carbonate (PPC), have a much higher lithium transfer coefficient compared to other commercially used systems, for example polyethylene oxide-based (PEO).
Reduction of Lithium Dendrite Formation
"Two mechanisms are conceivable," explains Grünebaum. "One is a partial decoupling of lithium ion mobility from slower polymer chain kinetics, and the other is a significant size-dependent slowing of anions by the polymer matrix." The findings will help to transfer the favourable ion transport properties of PEC and PPC to other promising polymer electrolytes in the future and greatly slow down diffusion-induced lithium dendrite formation.
Study Available in Physical Chemistry Chemical Physics
The detailed results of their study were published by the researchers Dr Anna Gerlitz, Dr Diddo Diddens, Dr Mariano Grünebaum, Prof. Dr Hans-Dieter Wiemhöfer, Helmholtz Institute Münster (HI MS; IEK-12) of Forschungszentrum Jülich, Prof. Dr Andreas Heuer, Helmholtz Institute Münster and Institute for Physical Chemistry of the University of Münster, and Prof. Dr Martin Winter, Helmholtz Institute Münster and MEET Battery Research Center, as an open access article in the journal Physical Chemistry Chemical Physics.
