Theory Developed to Understand the Correlation of Ions
New hydrodynamic theory can be used to predict the statistically correlated motion of two ions as a function of their distance.
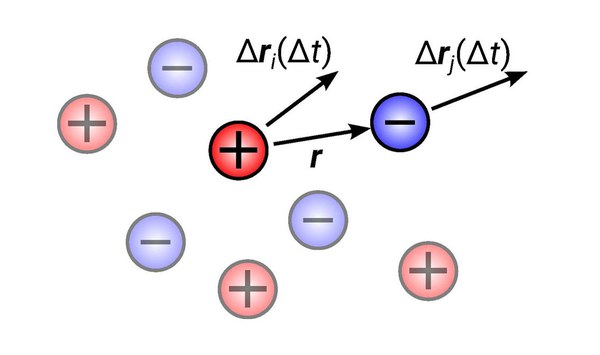
03 July 2023 – In their theoretical work, Dr Diddo Diddens from Helmholtz Institute Münster (HI MS; IEK-12) of Forschungszentrum Jülich and Prof. Dr Andreas Heuer from the Helmholtz Institute Münster and the Institute of Physical Chemistry of the University of Münster investigated the central question of the extent to which ions in liquid electrolytes move statistically correlated, i.e. together, in one direction. With this knowledge, the influence of individual factors, such as ion pairs on conductivity, can be better determined.
Neighbouring Ions Move in the Same Direction
It is often assumed that two ions with the same charge avoid each other due to mutual repulsion and thus move in opposite directions. Now the scientists were able to show that also two neighbouring ions with the same charge move in the same direction on average.
"This counterintuitive behaviour can be explained by the fact that the electrolyte, as a liquid, is incompressible, unlike gases. Therefore, a single ion can only move as its molecular environment allows," Diddens explains.
In contrast, the electrostatic interactions - repulsion of equally charged ions, attraction of unequally charged ions - primarily influence the local arrangement of the ions, but less the statistically correlated movement of neighbouring ions.
Hydrodynamic Theory for Predictions of Ion Movements
Molecular dynamics simulations are often used in battery research to determine how strongly correlated individual ions move in certain electrolytes. However, the movements are often difficult to interpret. Diddens and Heuer have now developed a new theory that predicts how much two ions move statistically correlated (together) depending on their distance.
With the help of the theory, the influence of individual factors, such as ion pairing on conductivity, can be better determined. The hydrodynamic effects are universal in liquids. Thus, they occur in many electrolytes, including certain polymers.
More Efficient Ion Transport for Novel Electrolytes
Determining the collective ion dynamics helps to understand how efficiently an electrolyte can transport a certain type of ion, for example lithium ions in lithium-ion batteries, and thus improve the fast-charging behaviour of a battery cell, among other things. The knowledge can be used for the design of new types of electrolytes.
Study Published in Journal of Chemical Physics
The detailed results of their study have been published by the researchers Dr Diddo Diddens from Helmholtz Institute Münster (HI MS; IEK-12) of Forschungszentrum Jülich and Prof. Dr Andreas Heuer from the Helmholtz Institute Münster and the Institute for Physical Chemistry of the University of Münster in the Journal of Chemical Physics. It is available for download free of charge here.
