Unexpected Cause of Capacity Loss in Lithium Metal Batteries Identified
New study shows: Local dry-out of pores and the formation of resistive intermediate layers at critical electrolyte amounts is the cause of “cell death”.
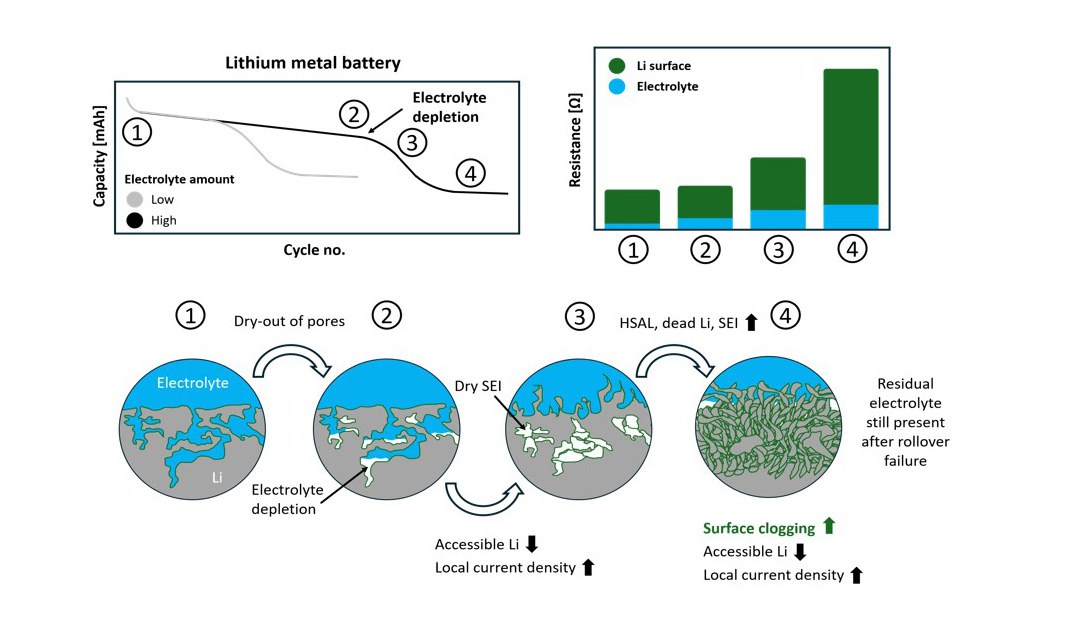
06 October 2025 – Researchers at Helmholtz Institute Münster (HI MS) of Forschungszentrum Jülich and the MEET Battery Research Center at the University of Münster have gained fundamental insights into the causes of capacity loss in lithium metal batteries in a collaborative project. The reason for premature cell failure is the formation of a resistive interphase on the anode, in particular at low electrolyte amounts: Even after so-called “cell death”, there is still enough electrolyte present in the rest of the cell to maintain sufficient ionic conduction.
Local Dry-Out With a Major Impact
The study shows that small amounts of electrolyte dry-out in the pores of the lithium electrode is crucial. This reduces the electrochemically active surface area, which increases the local current density. The effect promotes the uneven deposition of moss-like lithium (High Surface Area Lithium, HSAL). A resulting resistive intermediate layer of inactive SEI (Solid Electrolyte Interphase) and dead lithium drastically increases cell resistance, leading to accelerated capacity loss. Consequently, re-adding electrolyte after the end-of-life does not restore the capacity; but replacing the lithium anode does.
New Perspective for Research
While the dry-out of the electrolyte and the formation of dry pores had already been described in the literature, the study provides the first evidence that it is precisely this mechanism, in combination with HSAL formation and resistive interphases, that decisively determines cell death – and not electrolyte depletion itself.
This opens up new perspectives for the targeted development of electrolyte solutions for lithium metal batteries. The aim is to achieve an optimal balance between sufficient lithium passivation and a stable electrolyte quantity in order to optimize the compromise between energy density and the service life of future high-energy batteries.
Why Lithium Metal Batteries?
Lithium metal batteries are considered a promising addition to the lithium-ion batteries that dominate the market today. Thanks to the significantly higher gravimetric and volumetric capacity of lithium metal as an anode material, they offer potential for powerful energy storage in a compact design. A detailed understanding of the underlying degradation mechanisms is therefore crucial for developing tailor-made and long-lasting electrolyte solutions.
Cooperation Between Helmholtz Institute Münster and Meet Battery Research Center
Interdisciplinary cooperation was crucial in elucidating the complex interactions in lithium metal cells. While the MEET Battery Research Center contributed its expertise in the field of materials, Helmholtz Institute Münster focused on the properties of liquid electrolytes. Together, they succeeded in deciphering the causes of accelerated cell death. Building on these findings, further joint projects are already planned with the aim of investigating additional failure mechanisms and developing technical solutions.
Publication
The detailed results were published as an open access article in the journal Advanced Energy and Sustainability Research.
Authors involved:
- Dominik Weintz, Helmholtz Institute Münster (HI MS) of Forschungszentrums Jülich
- Dr Lukas Stolz, MEET Battery Research Center of the University of Münster and Stanford University
- Dr Marlena Bela, MEET Battery Research Center
- Tobias Hinz, Helmholtz Institute Münster
- Prof. Dr Martin Winter, Helmholtz Institute Münster and MEET Battery Research Center
- Dr Isidora Cekic-Laskovic, Helmholtz Institute Münster
- Dr Markus Börner, MEET Battery Research Center
- Dr Johannes Kasnatscheew, MEET Battery Research Center
