Evaluating the determination of solid-phase diffusion and reaction-rate constant for Li-ion batteries
DOI: 10.1016/j.est.2025.117628
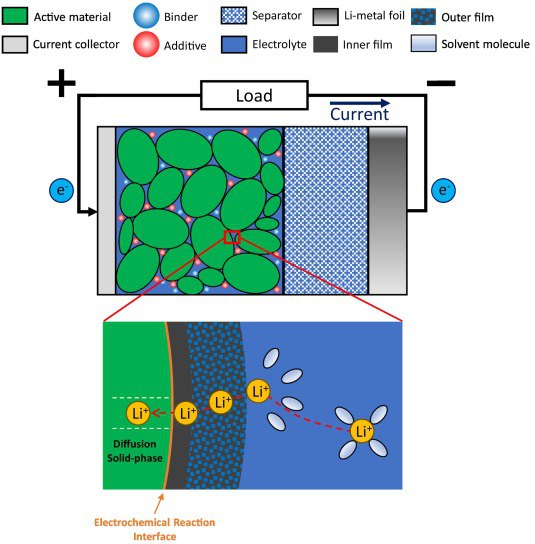
Abstract: Physics-based models are important tools for improving Li-ion battery performance, with their accuracy heavily dependent on key parameters such as the solid-phase diffusion coefficient (Ds) and reaction-rate constant (k0). In this work, galvanostatic intermittent titration technique (GITT) and potentiostatic intermittent titration technique (PITT) measurements were conducted on half-cells with a LiNi0.4Co0.6O2 (NC46) electrode from a commercial battery. Ds and k0 were determined using Weppner and Huggins' 1977 analytical method and physics-based optimization with the DFN model. These parameters were then implemented into the DFN model and validated under constant current with varying current densities and dynamic cycles. The combination of GITT measurements with the DFN model achieved the highest accuracy (average RMSE of 12.6 mV), while the analytical approach showed lower accuracy, especially with GITT measurements (average RMSE of 53.7 mV). Findings indicate that the widely used analytical approach in combination with GITT measurements may be unsuitable for accurately estimating Ds and k0 due to inherent limitations and assumptions, as demonstrated here for the NC46 material. The proposed DFN model approach in combination with GITT measurements demonstrated high accuracy and versatility in determining Ds and k0 across all lithiation levels. A sensitivity analysis further revealed that using the initial relaxation region of the GITT pulse is optimal for estimating with the analytical approach.
Cite as: Haider, A., Raijmakers, L.H.J. et al. Evaluating the determination of solid-phase diffusion and reaction-rate constant for Li-ion batteries. J. Energy Storage 132, 117628 (2025). https://doi.org/10.1016/j.est.2025.117628
