Hydrogen-Bond Network-Mediated Solvation Engineering Enables Synchronous Optimization of Zinc Anodes Kinetics and Iodine Cathodes Redox
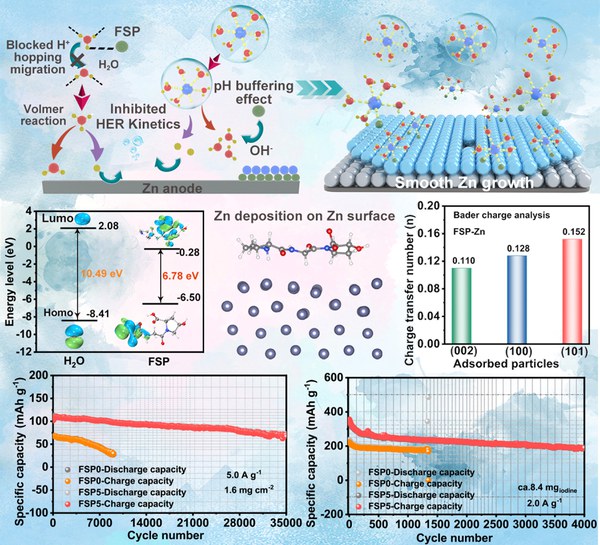
Abstract: The stability and durability of Zn anodes are compromised by the synergistic effects of H2O-mediated side reactions, unstable interfacial charge transfer, electrolyte decomposition, and dendrite formation induced by the space-charge layer. To overcome these challenges, a polypeptide additive, fish scale protein (FSP), with diverse surface morphology, is introduced to strengthen the stability of Zn anodes through multilevel regulation. Comprehensive studies integrating structural characterizations, first-principles calculations, and molecular dynamics simulations reveal that this strategy reconstructs the hydrogen-bond (HB) network within the electrolyte, thereby reducing the activity of free H2O, enhancing thermodynamic stability, and accelerating charge-transfer kinetics in Zn anodes. Moreover, the modified electrolyte suppresses the Grotthuss proton conduction mechanism, significantly impeding proton transport to the Zn surface and attenuating the kinetics of the HER. Therefore, the Zn anodes exhibit excellent cycling stability (4200 h). Furthermore, the Zn||CPC ZHSCs with FSP demonstrate stable cycling over 35 000 cycles. Additionally, the interaction between FSP and polyiodide eliminates the shuttle effect of polyiodides, enabling Zn─I2 batteries with exceptional cycling stability under high-load conditions (8.4 mg I2 loading, 4000 stable cycles). This coordinates a multi-level control strategy, which provides a promising strategy for developing highly stable Zn anodes, ZHSCs, and Zn─I2 batteries.
Cite as: T. Wu, M. Xi, Y. Huang, X. Chen, Y. E. Durmus, H. Zhang, J. Ding, H. Tempel, R. Eichel, Z. Tan, Hydrogen-Bond Network-Mediated Solvation Engineering Enables Synchronous Optimization of Zinc Anodes Kinetics and Iodine Cathodes Redox. Adv. Funct. Mater. 2025, 2507187. https://doi.org/10.1002/adfm.202507187
