Raman Spectroscopy
Our group applies Raman spectroscopy for the research of energy materials such as battery electrodes or electro-catalysts. Raman spectroscopy probes the vibration of molecules and lattices and thus provides mainly qualitative chemical information but can also be used for quantitative analysis. Due to the flexibility of modern Raman spectrometers, the technique is predestined for in operando research, i.e. the study of materials changing during operation of a battery or electrolysis cell. In addition, a Raman spectrometer is often coupled with a light microscope, which enables the scanning of energy materials to identify, for example, sample inhomogeneities before and after operation.
In light of modern devices, Raman spectroscopy is an increasingly popular method for the qualitative and quantitative analysis of a variety of materials, but in particular for electrocatalysts and electrode components. The Raman technique probes the vibration of molecules and lattices by exploiting the so-called Raman effect, also known as the inelastic scattering of light. The basic idea is that a laser is shone on a sample, which excites its atoms and molecules to vibrate with a certain energy. That energy is specific to a certain molecule or crystal, and can be used to identify and distinguish different chemical materials. The Raman spectrometer determines this energy by comparing the energy of the laser before and after it has hit the sample in question.
Our Raman spectrometers are also combined with light microscopes, which add spatial information on top of the chemical data. This enables the scanning of surfaces such as electrodes before and after operation (so called ‘post-test’), as different electrochemical processes may alter the material inhomogenously. The goal of such post-test studies is to investigate how the components of an electrochemical cell alter or degrade during operation, so that weak spots may be identified and eliminated, ultimately resulting in batteries or electrolysers with a longer lifetime.
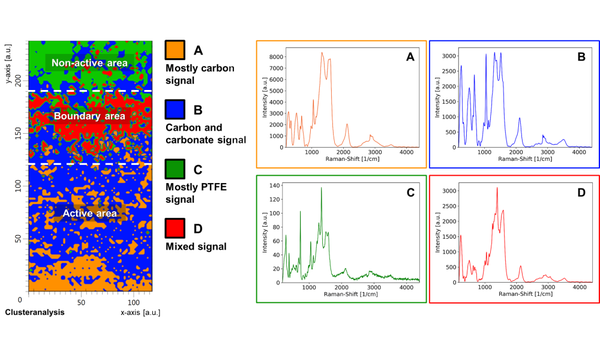
A research example is the study of post-test gas diffusion electrodes used for the electrochemical reduction (i.e. recycling) of carbon dioxide (doi: 10.1149/1945-7111/ab8ce1). Here, the electrode made of silver and PTFE binder turned black after operation, and strong carbon Raman signals were detected on its surface. At first glance, it was suspected that the PTFE binder degraded to elemental carbon during operation, which would ultimately result in failure of the electrolysis cell. Closer inspection however revealed that this was not the case, and instead an electrochemical roughening of the silver electrode took place. Such a rough silver surface can cause a dramatic enhancement of small chemical traces such as carbon by means of the SERS effect (Surface Enhanced Raman Scattering). Therefore, no PTFE was degraded, but instead the structure of the silver electrode surface was changed during electrolysis.
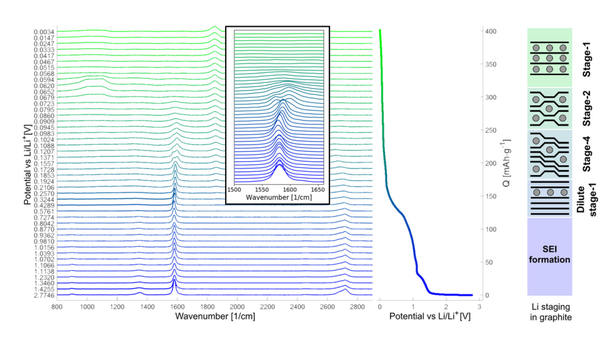
A major advantage of Raman spectroscopy is that it requires only minimal sample preparation. Thus, battery or other electrochemical cells can be studied in a comparatively uncomplicated manner by including a glass window in the cell construction. Studying a cell during the discharging and charging cycles, i.e. during operation, is denoted as an ‘in-operando’ technique. In operando Raman spectroscopy of lithium-ion batteries in particular reveals a great number of details about the transport of lithium cations between anode and cathode. In case of the common graphite anode, lithium ions are ‘intercalated’ into the graphite lattice, meaning they are inserted between sheets of hexagonal carbon lattice. Raman spectroscopy can be employed to follow this intercalation process, and even tell how many layers of these carbon sheets are between these inserted lithium ions. In one of our research studies (doi: 10.1002/elsa.202100068) we aimed to extract new information from the resulting in operando Raman spectra by not only analysing height and position of the peaks, but also their width and shape. This in-depth look provided insights in which way and order lithium ions are inserted into the graphite lattice, which may enable the synthesis of new, more durable materials.


