New Research Breakthrough in Lithium Metal Batteries
Capacity losses can now be precisely differentiated for the first time to extend service life
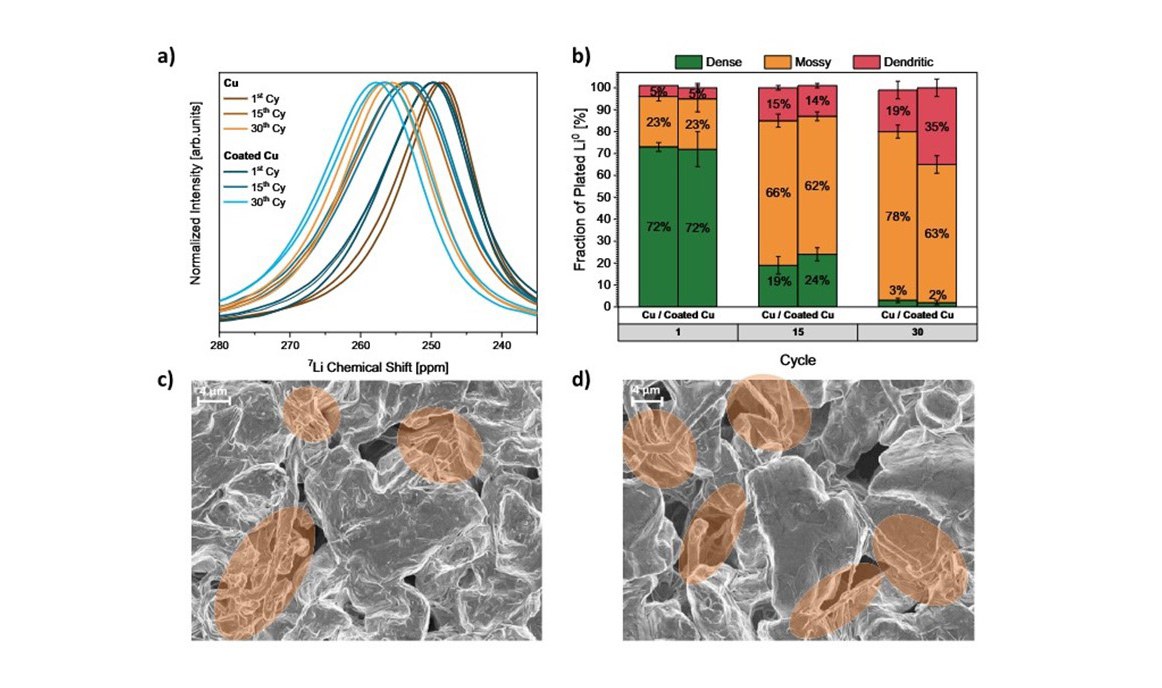
23 October 2025 – How can the service life of lithium metal batteries be specifically extended? A German-Taiwanese research team involving Helmholtz Institute Münster (HI MS) of Forschungszentrum Jülich has investigated this key question. In their new publication, the scientists show how different mechanisms of capacity loss can be precisely and non-destructively differentiated from one another for the first time – a decisive step towards more durable and powerful energy storage devices.
Capacity Losses Become Visible Through NMR Spectroscopy
The service life of batteries is significantly limited by irreversible processes at the negative electrode. In lithium metal batteries, two mechanisms in particular are responsible for this: the formation of electrically insulated “dead lithium” deposits and the passivation of the electrode.
Using high-resolution NMR spectroscopy, the research team has succeeded in quantitatively distinguishing between these two effects at different points in the battery life cycle – without causing any damage. This makes it possible for the first time to understand which of the two processes contributes to degradation and to what extent. This new methodology opens up the possibility of improving materials and cell concepts in a more targeted manner.
Alloy-Forming Coating as a Key Technology
The study focused in particular on comparing novel electrode materials. It was shown that an alloy-forming coating on copper significantly reduces the formation of dead lithium – by around 60 per cent after 30 charge and discharge cycles compared to uncoated copper electrodes.
“It is noteworthy that the previous explanation, namely a more compact deposition with a smaller surface area, could not be confirmed. The morphology of the deposited lithium hardly differed between deposition on a coated and an uncoated copper electrode,” explains PD Dr Gunther Brunklaus from Helmholtz Institute Münster. “Instead, impedance measurements show that the coating improves the ionic transport properties between the electrode and lithium. This allows the lithium to dissolve more completely, which is a key factor in the reversibility of the system.”
Why Lithium Metal?
Lithium metal is considered a key material for batteries with particularly high energy density, such as those used in electric vehicles or aviation applications. However, practical use has been limited to date due to challenges in the stable deposition and redissolution of lithium.
Losses are particularly noticeable in so-called “anode-free” cell concepts, which do not use an excess lithium metal electrode. This cell architecture is ideal for investigating the fundamental processes of electrodeposition and offers enormous potential for the development of more efficient and longer-lasting energy storage devices.
International Cooperation as a Factor for Success
The project was carried out in close cooperation with Prof. Dr Bing-Joe Hwang's working group at the National Taiwan University of Science and Technology (NTUST). This collaboration, which has been in place since 2017, combines material development on the Taiwanese side with the analytical expertise of Helmholtz Institute Münster. This ideal synergy is now being continued in the third funding phase of the joint research project “LiBEST”.
Study Published in the Journal Nature Communications
The researchers have published the detailed results of their study as an open access article in the journal Nature Communications.
Researchers involved:
• Lennart Wichmann – formerly Helmholtz Institute Münster of Forschungszentrum Jülich
• Shi-Kai Jiang – formerly Nano-electrochemistry Laboratory, National Taiwan University of Science and Technology
• Johannes Helmut Thienenkamp – Helmholtz Institute Münster
• Marvin Mohrhardt – formerly Helmholtz Institute Münster
• Prof. Dr Bing Joe Hwang – National Taiwan University of Science and Technology
• Prof. Dr Martin Winter – Helmholtz Institute Münster, MEET Battery Research Center of the University of Münster
