Automated synthesis of PET tracers
The steadily growing clinical interest in molecular imaging techniques such as positron emission tomography (PET) is also leading to an increasing demand for new, easily reproducible synthesis methods for the production of b+ emitter-bearing radioactive drugs (PET tracers), which can be carried out with very high levels of radioactivity (> 50 GBq). At the same time, these synthesis processes as a whole must meet the high standards and requirements of “Good Manufacturing Practice” (GMP). In order to comply with official regulations, particularly with regard to sterility and purity of the products, as well as to protect the manufacturing operators from ionizing radiation, the radiopharmaceuticals are synthesized with the aid of synthesis modules located in lead-shielded cells (so-called hot cells). Of course, the latter presupposes that such synthesis processes and equipment can be automated and remotely controlled by computer. The most common models currently available, which are particularly suitable for the above-mentioned purposes, can be divided into two main categories: Systems with a fixed predefined tubing design, and systems that use so-called disposable cassettes.

Depending on the requirements, both the one and the other can be advantageous or disadvantageous. For this reason, both systems are used in radiopharmaceutical production at the INM-5. While commercially available models were used for the cassette systems, these systems with a fixed design are, with a few exceptions, completely designed, developed and built by INM-5 itself, including the necessary software, in the FZJ workshops. The following figure shows an example of such a synthesis apparatus (right in the picture) including the corresponding P&I flow diagram (left in the picture).
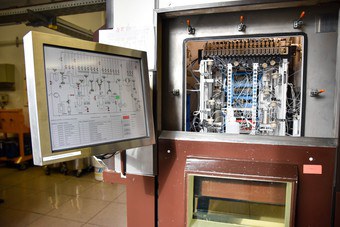
Sequence of an automated synthesis using the example of [18F]FET
Once the synthesis unit has been cleaned, it is filled with all the starting materials required for the production of [18F]FET in accordance with a previously defined and established standard operating procedure (SOP). Once all further synthesis preparations have been completed, the lead box of the “hot cell” is closed manually. At the same time as the synthesis preparations, the required 18F- is produced by irradiation of approx. 1.5 mL H218O with 16 MeV proton energy at the in-house baby cyclotron by the nuclear reaction18O(p,n)18F. [18F]fluoride is then transported into the synthesis apparatus by means of a helium stream.
Synthesis of O-(2-[18F]Fluorethyl)-l-tyrosin
After starting the synthesis, [18F]fluoride is first fixed on an anion exchanger, then eluted with an aqueous K2CO3 solution and fed into the reactor of the synthesis apparatus. The resulting fluoride ion is a weak nucleophile due to its high degree of solvation and high charge density. In order to increase the reactivity of 18F- to such an extent that nucleophilic substitutions are possible, so-called phase transfer catalysts (e.g. Kryptofix-2.2.2) must be added and water removed.
To remove the water, the solution is azeotropically distilled several times after the addition of acetonitrile.
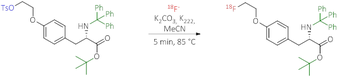
By adding triphenylmethylamino-3-(4-(2-(toluene-4-sulfonyloxy)ethoxy)phenyl)propionic acid tert-butyl ester, a protected precursor of the desired product is obtained within 5 min at 85 °C, which can then be converted into the crude product solution of [18F]FET by cleavage of the protecting groups (shown in green) in the presence of HCl at 100 °C.

Chromatographic purification and bottling
After deprotection, the crude product solution is cooled to room temperature, neutralized with aqueous sodium hydroxide solution and purified by preparative HPLC.
The product-containing fraction is then transferred to a collection vessel, the final activity and volume are determined and transferred to sterile ampoules via a sterile membrane filter using the automatic filling system. The manufactured product is usually divided into four portions (quality control sample, patient sample, sterile test sample, reserve sample). The sterile test sample and the reserve sample are initially stored in the decay vault and processed further later. The patient sample is packed by the radiation protection officer in a suitable transport box, sealed with a seal and sent to the predetermined recipient.
Quality control and release of radiopharmaceuticals at INM-5
Parallel to the transfer and transportation process, the quality control sample is tested in accordance with the test instructions. Various analytical methods are used here. For example, GC is used to check for residual solvents, HPLC provides information on radiochemical and chemical purity and the Lumulus test can be used to determine the endotoxin content.
The results of the analyses performed are transferred to an analysis protocol and checked by the quality control manager. The manufacturing protocol and the analysis protocol are then handed over to the qualified person together with all the raw data. This person carries out a document check. If all specification requirements are met and there are no quality-relevant deviations, the product batch is released for use by the signature of the qualified person on the analysis report.
