18F-Labeled azaisatoic anhydrides as versatile prosthetic groups for indirect radiofluorination
Abstract
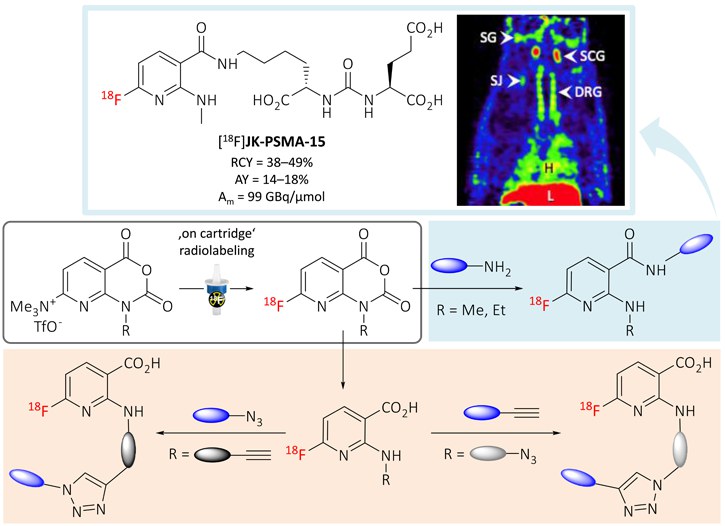
To enable 18F-labeling of sensitive molecules under mild conditions, 1-alkylamino-7-[18F]fluoro-8-azaisatoic anhydrides ([18F]AFAs) were developed as new prosthetic groups for indirect radiofluorination. The [18F]AFAs were efficiently prepared within 15 min using readily accessible precursors and could be conjugated with a range of amines and alkyne- or azide-functionalized precursors to afford the corresponding 18F-labeled conjugates in high radiochemical conversions of up to 98%. The practical utility of the new prosthetic groups was confirmed by successful radiosynthesis of three 18F-labeled PSMA ligands with excellent in vivo stability and, in one case, promising imaging properties.
Gröner, B.; Willmann, M.; Donnerstag, L.; Urusova, E.; Neumaier, F.; Humpert, S.; Endepols, H.; Neumaier, B.; Zlatopolskiy, B. D.
Journal of medicinal chemistry 2023, 66 (17), 12629-12644.