Fast and efficient 18F-labeling via Pd-catalyzed S-arylation in aqueous medium
Abstract
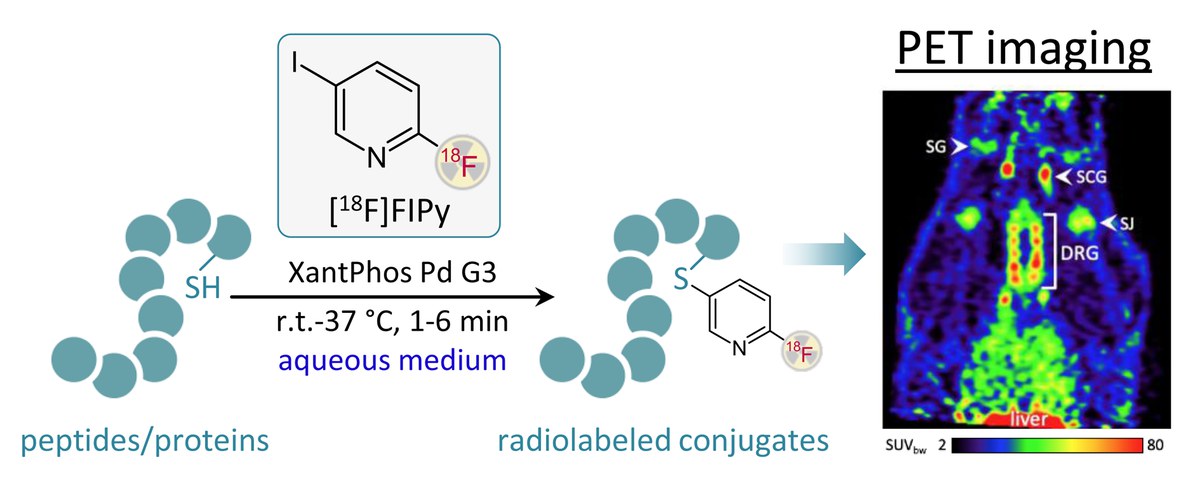
A novel indirect radiolabeling method for the radiofluorination of thiol-containing substrates based on Pd-catalyzed S-arylation was developed. The prosthetic group, 2-[18F]fluoro-5-iodopyridine, is easily accessible through a simple, minimalist radiofluorination technique. The versatility and practicality of the method were demonstrated by the successful synthesis of a novel PSMA-specific PET tracer, as well as 18F-labeling of glutathione, the Aβ oligomer-binding RD2 peptide, bovine serum albumin, and PSMA I&S. The approach enables fast, efficient, and robust labeling, making it highly suitable for the development of new PET tracers targeting various biomolecular structures.
Humpert, S.; Omrane, M. A.; Urusova, E. A.; Gremer, L.; Willbold, D.; Endepols, H.; Krasikova, R. N.; Neumaier, B.; Zlatopolskiy, B. D., Rapid
18F-labeling via Pd-catalyzed S-arylation in aqueous medium.
Chem. Comm. 2021, 57 (29), 3547-3550.