Efficient Production of Carbonyl Sulfide in the Low-NOx Oxidation of Dimethyl Sulfide
Christopher M. Jernigan, Charles H. Fite, Luc Vereecken, Max B. Berkelhammer, Andrew W. Rollins, Pamela S. Rickly, Anna Novelli, Domenico Taraborrelli, Christopher D. Holmes, Timothy H. Bertram
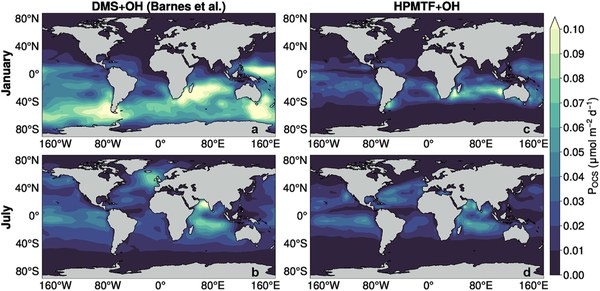
Abstract
The oxidation of carbonyl sulfide (OCS) is the primary, continuous source of stratospheric sulfate aerosol particles, which can scatter shortwave radiation and catalyze heterogeneous reactions in the stratosphere. While it has been estimated that the oxidation of dimethyl sulfide (DMS), emitted from the surface ocean accounts for 8%–20% of the global OCS source, there is no existing DMS oxidation mechanism relevant to the marine atmosphere that is consistent with an OCS source of this magnitude. We describe new laboratory measurements and theoretical analyses of DMS oxidation that provide a mechanistic description for OCS production from hydroperoxymethyl thioformate, a ubiquitous, soluble DMS oxidation product.
