Effect of the Alkoxy Radical Chemistry on the Ozone Formation from Anthropogenic Organic Compounds Investigated in Chamber Experiments
Michelle Färber, Hendrik Fuchs, Birger Bohn, Philip T. M. Carlsson, Georgios I. Gkatzelis, Andrea C. Marcillo Lara, Franz Rohrer, Luc Vereecken, Sergej Wedel, Andreas Wahner, and Anna Novelli
Abstract
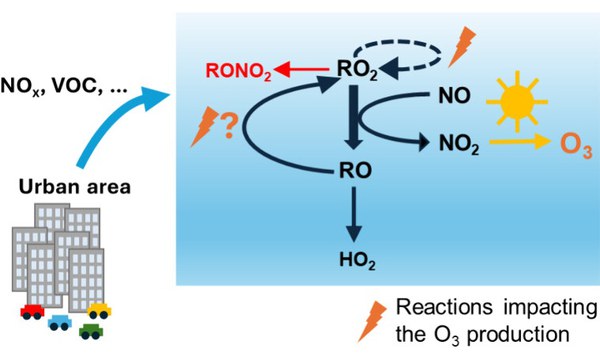
The photooxidation of five anthropogenic volatile organic compounds (propane, propene, isopentane, n-hexane, trans-2-hexene) at different levels of nitric oxide (NO) was investigated in the atmospheric simulation chamber SAPHIR, Forschungszentrum Jülich. Measured time series of trace gases and radical concentrations are compared to zero-dimensional box model calculations, based on the Master Chemical Mechanism (agreement within 30%) and complemented by state-of-the-art structure–activity relationships (SAR). Including RO2 isomerization reactions from SAR, validated with theoretical calculations, improves particularly the model–measurement agreement by ∼20% for n-hexane. The photooxidation of the chosen compounds generates different types of peroxy radicals (RO2) which produce HO2 after one or multiple RO2+NO reaction steps, depending on the formed alkoxy radical (RO). Measurements show that the HO2/RO2 ratio is up to ∼40% lower and the number of odd oxygen (Ox = O3+NO2) formed per OH+VOC reaction (𝑃(O𝑥)VOC) is up to ∼30% higher if RO regenerates RO2 instead of forming HO2 directly. Though, the formation of organic nitrates nearly completely compensates for the ozone production from the second NO reaction step for nitrate yields higher than 20%. Measured and modelled HO2/RO2 ratios agree well as does 𝑃(O𝑥)VOC, derived from measured/modelled radical concentrations and calculated from measured Ox.
