Urban Air Quality
Table of Contents
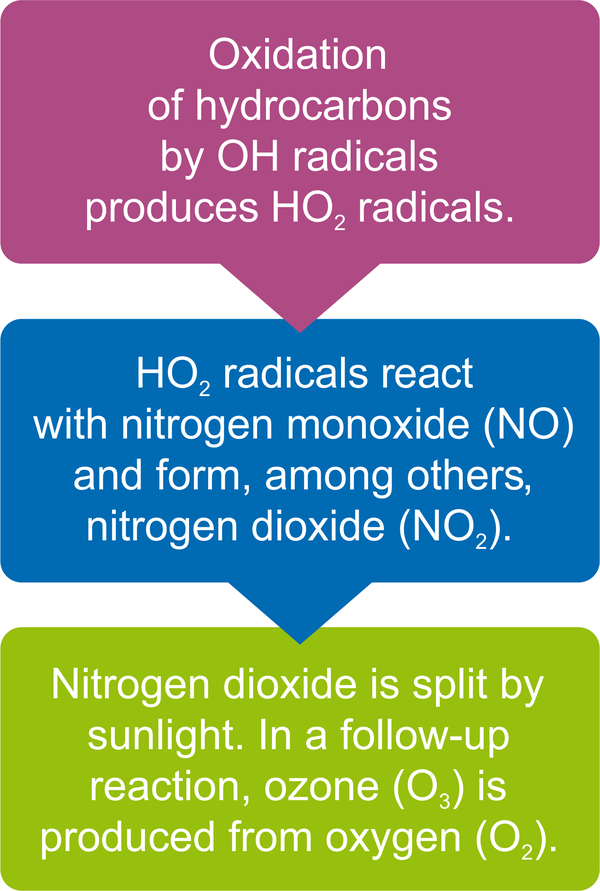
How does traffic exhaust lead to the ozone formation?
We have long been concerned with the complex factors regulating ozone formation in the lower layer (1-2 km) of our atmosphere. In order to understand how the traffic affects ozone production, we first consider the properties of gasoline and diesel vehicles.

We have determined with our measurements how much hydrocarbons (VOC) and nitric oxide concentrations in German city centers have declined over the last 20 years. With these results we have modeled the ozone production rate.
Hydrocarbon concentrations in inner cities have been lowered as a result of the catalysts. On the other hand, nitric oxide emissions have hardly been reduced by the many diesel engines. As a result, the nitrogen dioxide load in German inner cities remains unchanged too high.
Of course, nitrogen oxide concentrations have to be reduced. However, due to the lower ratio of hydrocarbons to nitrogen oxides, ozone is hardly produced in the cities anymore!
Both hydrocarbons and nitrogen oxides are involved in ozone formation. At low nitrogen oxide concentration levels, the more nitrogen oxides are present in the air, the more ozone is formed. In Germany's inner cities, however, the concentration of nitric oxide is so high that ozone formation is slowed down.
How has the ozone production rate changed over time?
Ozone formation in 1990 and now
Things have changed since in the 1990s catalysts for vehicles were introduced. In order to understand these changes, you should imagine what happens with an air package that travels from the city towards the forest ...
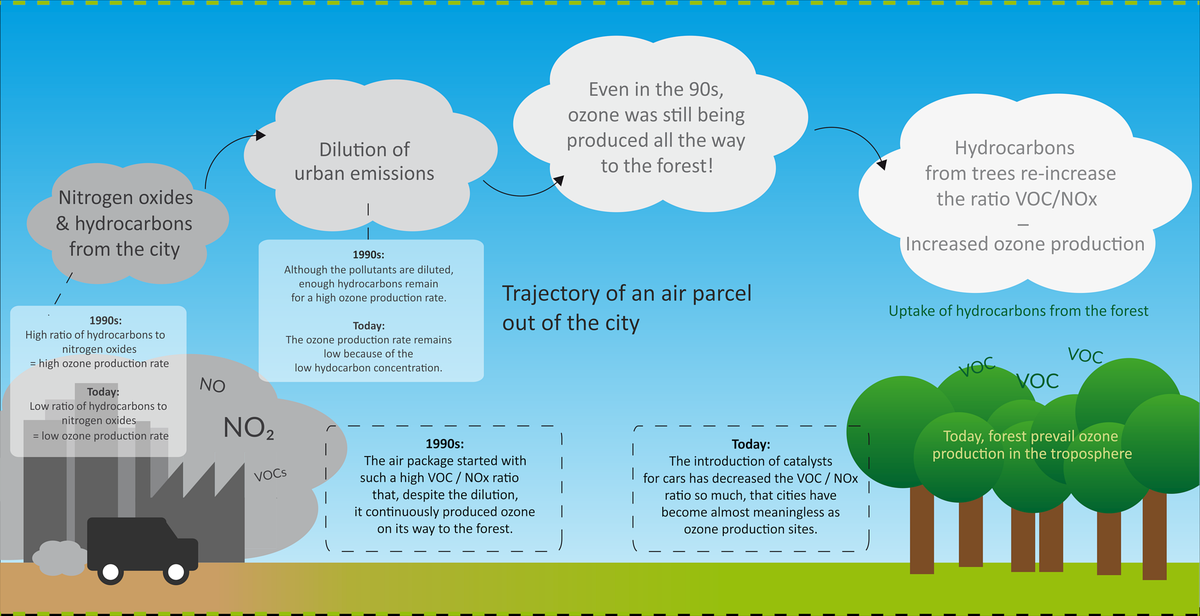
To put it simply, a high ratio of hydrocarbons to nitrogen oxides in the exhaust gas increases ozone production. In the 1990s, both the concentrations of nitric oxide and hydrocarbons in cities were very high - as did ozone production. When the air package moved towards the surrounding countryside, the pollutants were diluted; but they were sufficient for a consistently strong ozone production.
Today, thanks to the catalysts in the cities, hardly any hydrocarbons are emitted. Nitrogen oxide emissions have only slightly declined. The smaller ratio of hydrocarbons to nitrogen oxides leads to a significantly lower ozone production!
Naturally, nitrogen oxide emissions must also be reduced in the future since these are also harmful to the climate and health. However, with respect to ozone production, elevated nitrogen oxide concentrations are no longer problematic at the current hydrocarbon concentration level.
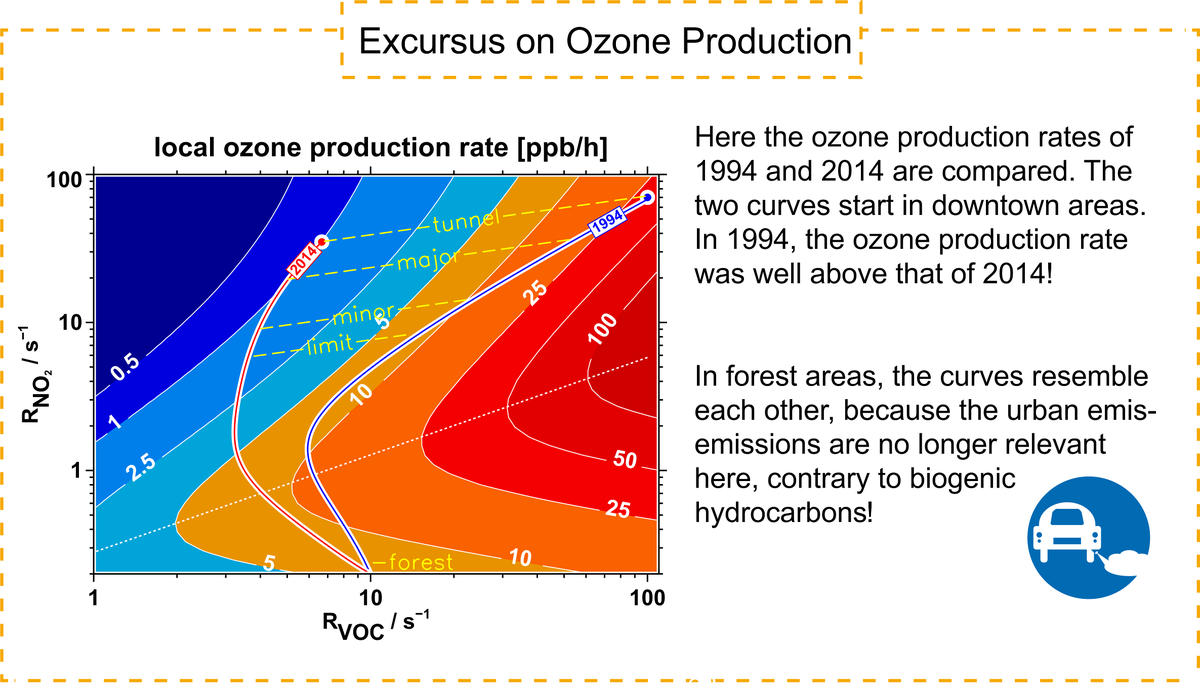
The ozone problem has not yet been solved worldwide. Our result of the reduced ozone production relates only to Germany. Although a similar trend is to be expected in Europe, there are also some countries with heavily obsolete or completely different vehicle fleets!
Nitrogen Oxides in German Inner Cities
From our research to date, the problem of the high ozone production rate in cities is, at present, solved. Now, the problems of nitric oxide emissions and fine dust loads must also be tackled. It should be ensured that old problems are not brought back to the back door or create new problems - therefore, air-chemical investigations are essential!
Particularly high particulate matter and pollutant loads occur when the exhaust fumes cannot be drained away from the city. This is especially an issue in cities with basin or valley topography or in the case of stable high-pressure weather conditions.
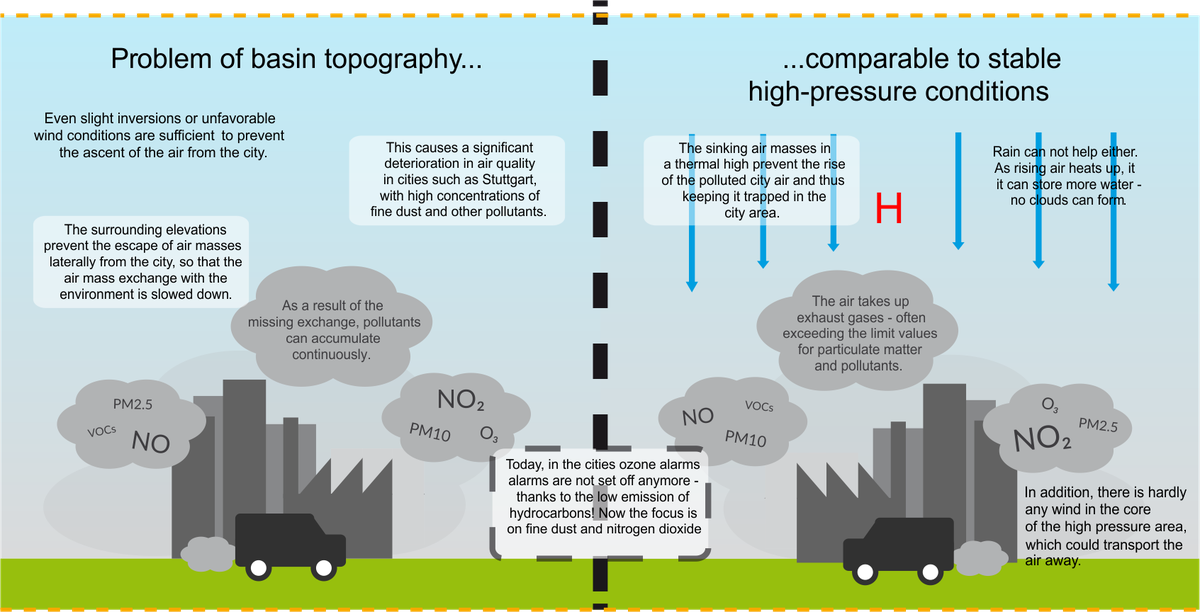
For towns that are surrounded by mountains (for example, Stuttgart), the city air cannot easily travel sideways towards fields and forests. If there is slight inversion or unfavorable wind conditions, an ascent of the air is also suppressed. The air stays trapped in the city for a long time and is continuously enriched with exhaust fumes.
A similar effect is produced by a so-called stable high-pressure weather situation: sinking air masses prevent the city air from ascending and push fumes back into the city. During such special weather conditions, alarms for fine dust and nitrogen oxides often occur. Thanks to modern catalyst technology, the earlier peak values for ozone are no longer reached, but particles and especially nitrogen oxides remain problematic.
What is done about it?
EU regulations have already addressed this problem and have set limits - this forces the automotive industry to react. However, when a new technology is developed, it usually takes about a decade until a large part of the car fleet is equipped with it.
Environmental zones are another measure to reduce the pollutant concentrations in the inner city. This can lead to an exchange of the vehicle fleet in the long term if residents have to buy a car with modern exhaust gas cleaning technology. However, there is also the risk of a shifting of the problem to the surrounding area. Sooner or later, pollutants have to be worked on themselves.
SCR catalytic converters for diesel engines are designed to meet the EU limit values for nitrogen oxides. The use ammonia, which reacts with nitrogen oxides to produce nitrogen and water. For the supply of ammonia, urea is used, is additionally fueled and split into ammonia at high temperature.
Where do we get into the game?
New research questions are coming up with new technologies. In the case of the SCR catalysts, for example, the question arises as to whether and how much ammonia can leak from the catalyst. In addition, the extremely toxic formed as the product of the decay of urea along with ammonia. Although isocyanic acid is believed to be removed by reaction with water, it is questionable whether this process happens completely.
In the future, we will continue to support the development of traffic emissions with our measurements. In order to avoid unwanted side-effects of new technologies, air-chemical investigations are useful already in the run-up to market launch.
