Motile Growing Tissues
Motile Tissues

Interestingly, cells can do both: they not only grow, but they also actively migrate. Combined, growth and motility lead to novel phenomena: Motile cells, even at confluency, move about, displaying swirl-like patterns. When a boundary is suddenly removed, the tissue invades the free space, and the interface undergoes a fingering instability. Particularly puzzling is the fact that growing Madin-Darby canine kidney (MDCK) colonies are not under pressure, but under tension. Using the same particle-based tissue simulation technique as in the growth simulations, extended them with a minimalistic motility model, reveals that a purely local motility alignement is sufficient to reproduce the experimentally observed patterns.
Further reading:
Alignment of cellular motility forces with tissue flow as a mechanism for efficient wound healing
Alignment of divisions with motility
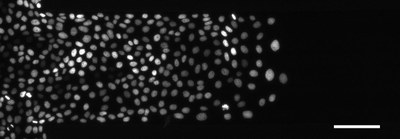
Cell division is an essential dynamic event in tissue remodeling during wound healing, cancer and embryogenesis. In collective migration, tensile stresses affect cell shape and polarity, hence, the orientation of the cell division axis is expected to depend on cellular flow patterns. We quantified the degree of orientation of cell division axes in migrating and resting epithelial cell sheets. We use microstructured channels to create a defined scenario of directed cell invasion and compare this situation to resting but proliferating cell monolayers. In experiments, we find a strong alignment of the axis due to directed flow while resting sheets show very weak global order, but local flow gradients still correlate strongly with the cell division axis. We compare experimental results with our mesoscopic particle based simulation model, and find very good agreement.
Further reading:
Alignment of cell division axes in directed epithelial cell migration
