NMR/MRI
Nuclear magnetic resonance (NMR) and Magnetic Resonance Imaging (MRI) - Systems for non-invasive measurements of plant structure and function
Our MRI installations are a non-invasive modality allowing to measure dynamic changes in structural and functional traits of plant and soils under realistic environmental conditions.
Below ground applications
MRI enables imaging of roots growing in soil and thus makes it possible to analyse whole root architecture in three dimensions (Fig 1). The method yields detailed information on root traits such as mass, length, diameter, tip number, branching angles, spatial distribution and growth rates (van Dusschoten et al., 2016). For belowground storage organs like sugar beet, additional traits such as internal structure or shape development can be measured (Metzner et al., 2014). To fit the root imaging setup, plants are typically grown in tubes of 8.1 cm inner diameter and 20-40 cm height, while the plant shoot may be up to 100 cm in height. The tubes are handled by a robotic system, enabling fully automated long-term measurement series of about 20 plants per day. Depending on the number of measurements per plant, we can run about 20-40 experiments per year.
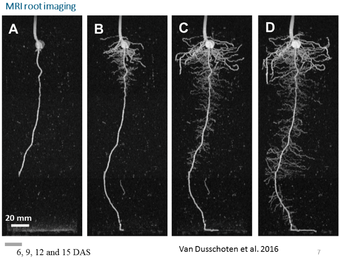
Examples of plant species of which root traits or bulky organs can be measured are maize, bean, barley, wheat, sugar beet or potato. The root structure of plants with very thin roots such as Arabidopsis cannot been measured.
The analysis of data gathered with MRI for one plant per pot is semi-automated by a software tool called NMRooting (van Dusschoten et al., 2016). Further, there is high flexibility to combine MRI with other methods (e.g. gas exchange or non-invasive monitoring of plant water distribution (Sydoruk et al. 2016).
Several scientific projects have been executed using the above MRI approach on roots. Soil density, for example, influences root length, diameter and tortuosity. Soil moisture depletion was observed to shift root growth to the wetter and deeper parts of the soil. Further, combining root imaging with soil water content imaging (with MRI) and a split root system enabled the differentiation between seminal and nodal root water uptake.
Above ground applications
For above ground studies, typical applications of high-field MRI on plants are the quantitative and non-invasive micro imaging of parameters such as water content, anatomy and sap flow. The most typical subjects are herbaceous plants of modest size, but if needed large trees can be handled as well. Our largest imager is able to accept potted trees of up to a height of 4.5 meters, and is able to measure xylem and phloem sap flow in stems of up to 8 centimetres in diameter.
One of the most unique features of MRI as applied on plants is that it allows the direct imaging of xylem and phloem sap flow inside the living plant. Recent applications of the MRI micro imaging and MRI flow imaging in our lab include imaging of xylem cavitation in grape stems and petioles (Hochberg et al 2016), of the effect of girdling on the xylem integrity of the tomato peduncle (Van der Wal et al, 2017), or the dynamics of phloem sap flow in gymnosperm trees (Liesche et al, 2015). An example of MRI micro imaging is a study on oak, in which quantitative micro imaging was successfully used to couple stem diameter changes, as measured by conventional point dendrometers, to changes in stem water content.
Mobile NMR sensors

NMR and MRI are unique in that they make it possible to quantitatively and non-invasively measure the presence and movement of water. Unfortunately, conventional equipment for NMR and MRI is usually large, expensive, and only suitable for use in a laboratory environment. Plants then need to be brought to the scanner, rather than the other way round.
In order to bring NMR technology to the field, we developed novel, small scale portable NMR devices that can be used in a sensor-like fashion (Windt and Blümler, 2015). In its simplest form, such NMR sensors consist of scaled down permanent magnets. They can be used, almost like a dendrometer, to measure dynamic changes in the absolute water content of fruit, leaves or stems (Lechthaler et al, 2016). By employing basic NMR relaxometry, the information that can be gained from these small scale NMR sensors can be extended, allowing the instantaneous, non-invasive measurement of changes in dry matter content as well. In this way, the development of yield (dry matter deposition) in terminal organs such as bean pods can be monitored, over a period of weeks if desired, without harvesting the plant (Fig. 2).
In addition to this, we are working to establish small scale, mobile NMR imagers. Our imagers are based on the same mobile permanent magnets that are used for the NMR sensors, but are fitted with imaging gradients and mobile, battery powered amplifiers to control them. Mobile imaging of plants is still in its infancy, but prototypes already have shown to be capable of low resolution MRI, as well as 1D xylem sap flow velocimetry (Windt and Blümler, 2015).
The 4.7 Tesla system
Wide-bore, 310 mm magnet, equipped with a 300 mT/m 210 mm wide gradient system. Different r.f. coils available.
- 63 mm quad birdcage
- 104 mm quad birdcage
- 170 mm quad birdcage
- 40 mm openable birdcage
- small r.f. coils manufactured locally
- Heavy objects up to 500 kg can be lifted into the magnet using a crane. Objects up to 200 kg can be positioned with a computer-controlled positioning system for repetitive studies with an accuracy of about 20 µm.
- environmental control systems complete the system.
1.5 T MRI am IBG-2: Pflanzenwissenschaften
The split coil magnet is unique in its kind. It is equiped with 50 mT/m 380 mm and 800 mT/m 120 mm planar gradient sets. Different r.f. coils are available
- 63 mm solenoid
- 140 mm solenoid
- several home-build splittable/wrappable solenoids
- Heavy objects, like trees with large pots/containers can be lowered into a pit a 2 meters deep. Objects up to 5 meter can be measured.
Selected Publications
Ceolin S, Schymanski SJ, van Dusschoten D, Koller R, Klaus J. 2025. Root growth dynamics and allocation as a response to rapid and local changes in soil moisture. Biogeosciences 22(3): 691-703. doi.org/10.5194/bg-22-691-2025
Coleman D, Windt CW, Buckley TN, Merchant A. 2023. Leaf relative water content at 50% stomatal conductance measured by noninvasive NMR is linked to climate of origin in nine species of eucalypt. Plant, Cell & Environment 46(12): 3791-3805. doi.org/10.1111/pce.14700
Merchant A, Smith MR, Windt CW. 2022. In situ pod growth rate reveals contrasting diurnal sensitivity to water deficit in Phaseolus vulgaris. Journal of Experimental Botany 73(11): 3774-3786. doi.org/10.1093/jxb/erac097
Pflugfelder D, Kochs J, Koller R, Jahnke S, Mohl C, Pariyar S, Fassbender H, Nagel KA, Watt M, van Dusschoten D. 2022. The root system architecture of wheat establishing in soil is associated with varying elongation rates of seminal roots: quantification using 4D magnetic resonance imaging. Journal of Experimental Botany. doi.org/10.1093/jxb/erab551
Kirschner GK, Rosignoli S, Guo L, Vardanega I, Imani J, Altmüller J, Milner SG, Balzano R, Nagel KA, Pflugfelder D, et al. 2021. ENHANCED GRAVITROPISM 2 encodes a STERILE ALPHA MOTIF–containing protein that controls root growth angle in barley and wheat. Proceedings of the National Academy of Sciences 118(35): e2101526118. doi.org/10.1073/pnas.2101526118
Meixner M, Tomasella M, Foerst P, Windt CW. 2020. A small-scale MRI scanner and complementary imaging method to visualize and quantify xylem embolism formation. New Phytologist 226(5): 1517-1529. doi.org/10.1111/nph.16442
Bouda M, Windt CW, McElrone AJ, Brodersen CR. 2019. In vivo pressure gradient heterogeneity increases flow contribution of small diameter vessels in grapevine. Nature Communications 10(1): 5645. doi.org/10.1038/s41467-019-13673-6
Van de Wal BAE, Windt CW, Leroux O, Steppe K. 2017. Heat girdling does not affect xylem integrity: an in vivo magnetic resonance imaging study in the tomato peduncle. New Phytologist 215(2): 558-568. doi.org/10.1111/nph.14610
Pflugfelder D, Metzner R, van Dusschoten D, Reichel R, Jahnke S, Koller R. 2017. Non-invasive imaging of plant roots in different soils using magnetic resonance imaging (MRI). Plant Methods 13(1): 102. doi.org/10.1186/s13007-017-0252-9
van Dusschoten D, Metzner R, Kochs J, Postma JA, Pflugfelder D, Buehler J, Schurr U, Jahnke S. 2016. Quantitative 3D Analysis of Plant Roots growing in Soil using Magnetic Resonance Imaging. Plant Physiology 170: 1176-1188. doi.org/10.1104/pp.15.01388
Metzner R, Eggert A, van Dusschoten D, Pflugfelder D, Gerth S, Schurr U, Uhlmann N, Jahnke S. 2015. Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: potential and challenges for root trait quantification. Plant Methods 11: 11. doi.org/10.1186/s13007-015-0060-z

Contact
- Institute of Bio- and Geosciences (IBG)
- Plant Sciences (IBG-2)
Room 202
Dr. Dagmar van Dusschoten
Head high-field MRI lab
- Institute of Bio- and Geosciences (IBG)
- Plant Sciences (IBG-2)
Room 228


