Neutrons decipher interplay between proteins and their hydration water
August 2, 2012
Proteins are the building blocks of life. They lend structure to both animal and plant tissue and catalyse metabolic processes inside cells or in cell membranes. In nature, protein molecules are always surrounded by a layer of water. An international research team has investigated the influence of this “hydration shell” on the function of proteins and discovered significant differences between different types of proteins, with the help of neutron experiments at the Jülich Centre for Neutron Science. The results are published in the current edition of the international scientific journal “Biophysical Journal“(DOI: 10.1016/j.bpj.2012.05.027).Proteins are the building blocks of life. They lend structure to both animal and plant tissue and catalyse metabolic processes inside cells or in cell membranes. In nature, protein molecules are always surrounded by a layer of water. An international research team has investigated the influence of this “hydration shell” on the function of proteins and discovered significant differences between different types of proteins, with the help of neutron experiments at the Jülich Centre for Neutron Science. The results are published in the current edition of the international scientific journal “Biophysical Journal“(DOI: 10.1016/j.bpj.2012.05.027).
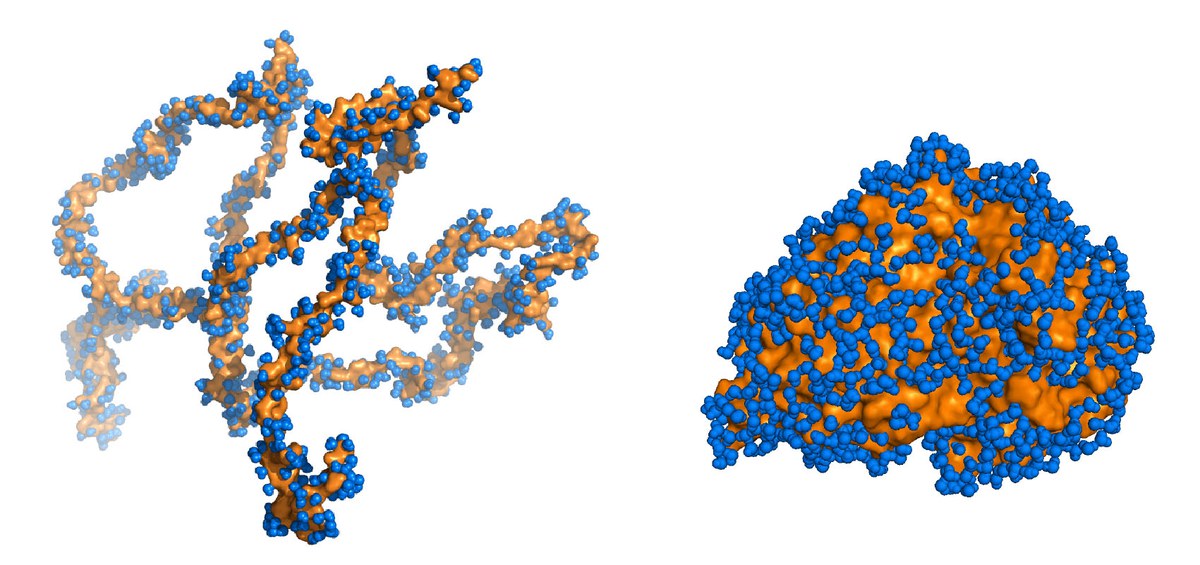
Proteins are made up of chains of amino acids arranged in an exact pre-determined sequence. Most proteins can only fulfil their intended function when these chains are folded into a convoluted cluster in the right way for the individual protein. However, the tau protein, which stabilizes transport paths in biological cells, is only partially folded. Furthermore, in order to achieve even this partially folded state, it needs a designated counterpart, the microtubules. Therefore, tau proteins belong to a group comprising around 30 % of known proteins in cells with nuclei with no clearly defined three-dimensional folds. Unfolded tau proteins tend to build up as deposits and destroy cells. Such deposits are found in the brains of those suffering from dementia, for example, Alzheimer patients.
Much is already known about the way in which properly ordered, folded proteins function. Water plays an important role here, as it supports the motions of amino acids and makes the correct folding and functioning of the protein possible. It attaches itself to hydrophilic, water-friendly elements in the proteins using hydrogen bonds and forms so-called hydration shells. Very little is known about the functionality of disordered proteins. This is due to the fact that X-ray crystallography – the standard method of determining the structure of a protein molecule – does not work very well with these proteins, and motions rather than structure play, in comparison, a far greater role here than in ordered proteins.
Now, for the first time, a research team made up of scientists from France, the USA, Australia and Germany have investigated the motions of the tau protein and its hydration shell as representative of disordered proteins, and compared them with ordered proteins from cell plasma and the cell membrane. Neutron experiments performed using a JCNS spectrometer at the research neutron source Heinz Maier-Leibnitz (FRM II) in Garching near Munich and on instruments at the Institut Lau-Langevin (ILL) in Grenoble, France, made it possible to perform the measurements.
“Our experiments show that the role of the hydration shell evidently differs markedly depending on the types of protein involved”, explains Dr. Martin Weik from the Institut de Biologie Structurale in Grenoble. As expected, physicists found greater motional flexibility in the tau proteins compared with folded protein molecules. However, the discovery that the tau protein and its hydration shell move together in much stronger unison than folded plasma proteins do, and that these in turn move more strongly than membrane proteins, came as a surprise.
“We hope that over the long term, further studies can provide us with information which can be used in the development of new medicines,” says Weik. As so little is known about the function of disordered proteins, up to now there has been no effective strategy employed in the search for appropriate sites for drug intervention. Researchers have already begun to analyse the pathogenic form of tau proteins using neutrons. “Neutrons are ideally suited to study the functionality of disordered proteins, as their motions can be measured in high resolution”, says Dr. Joachim Wuttke from the Jülich Centre for Neutron Science (JCNS) in Garching, who supports research scientists in their studies using the Jülich instrument.
Furthermore, by employing a special method, it is possible to selectively observe just the motions of the protein’s amino acids or just the water molecules in the shell, as neutrons detect hydrogen molecules in water and in proteins. By replacing normal hydrogen with heavy hydrogen, which is not so easily detected by neutrons, either the protein in the sample or the surrounding hydration shell can then be easily identified after staining. “That is only possible with neutrons”, explains Wuttke.
Original publication:
Dynamical Coupling of Intrinsically Disordered Proteins and Their Hydration Water: Comparison with Folded Soluble and Membrane Proteins; Gallat et al.;
Biophysical Journal, Volume 103, Issue 1, 3 July 2012, Pages 129–136; DOI: 10.1016/j.bpj.2012.05.027
Further information:
Jülich Centre for Neutron Science (JCNS): www.fz-juelich.de/jcns/
Forschungs-Neutronenquelle Heinz Maier-Leibnitz (FRM II): www.frm2.tum.de
Institut Laue-Langevin (ILL): www.ill.eu/
Institut de Biologie Structurale: www.ibs.fr
Contact:
Dr. Joachim Wuttke, Forschungszentrum Jülich, JCNS-Außenstelle am FRM II,
Garching, Tel. 089 289-10715, E-Mail: j.wuttke@fz-juelich.de
Dr. Martin Weik, Structural Protein Dynamics Research Team Institut de Biologie Structurale,
Grenoble, France, Tel: + 33 4 38 78 95 80, E-Mail: weik@ibs.fr
