Advanced Molecular Simulations On JUWELS Booster Pave The Way For Future HIV-1 Treatments
The global HIV-1 pandemic, with over 40 million infections, illustrates the virus's remarkable adaptation to humans. It originated from simian immunodeficiency viruses (SIV) in chimpanzees and gorillas, with HIV-1 M being the pandemic strain. There are other non-pandemic strains, HIV-1 N, O, and P, found in a few West African individuals.
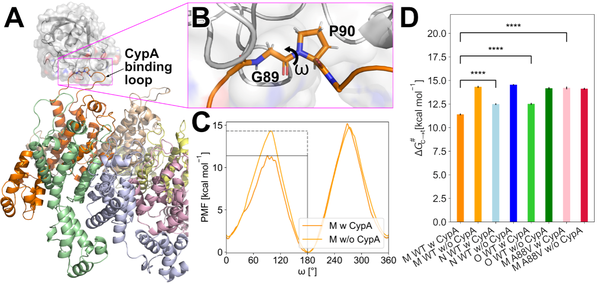
(A) Overview of the simulated complex. CYPA (shown in gray) binds to the CYPA binding loop (orange) on the surface of the HIV-1 capsid. (B) Close-up view of the CYPA binding loop. The ω dihedral between residues G89 and P90 is marked. (C) Potential of mean force (free energy profile) along the ω dihedral of G89-P90 in HIV-1 M wild type with or without CYPA. (D) Barrier heights (ΔG#t->c) from the potential of mean force computations for the cis/trans isomerization of the ω angle between G89 and P90. The HIV-1 types and variants are sorted according to the decrease of ΔG#t->c in the presence of CYPA.
Recent research by Prof. Dr. Holger Gohlke (IBG-4, Forschungszentrum Jülich and Heinrich Heine University Düsseldorf) and Prof. Dr. Carsten Münk (University Hospital Düsseldorf) uncovered a new understanding of how HIV-1 M adapts to human cells. Human cells defend against retroviruses, like SIV, using the TRIM5α protein. However, HIV-1 M avoids this defense by binding to another protein, cyclophilin A, a trans-cis-isomerase, which suppresses TRIM5α binding.
For this, computationally intense umbrella sampling molecular dynamics simulations of HIV-capsid protein/cyclophilin A complexes were performed on the JUWELS Booster module, exploiting the excellent performance of the AMBER molecular simulation code on GPUs. Followed by configurational free energy computations, these computations indicated that capsid residue 88 can affect trans-to-cis isomerization patterns on the capsids of the tested viruses. These differential CYPA usages by pandemic and non-pandemic HIV-1 suggest that the enzymatic activity of CYPA on the viral core might be important for its protective function against human TRIM5α.
The study identifies a potential vulnerability in HIV-1, offering hope for new drugs. By suppressing the binding of cyclophilin A to the virus, researchers may develop drugs to combat HIV-1. Published in "The Proceedings of the National Academy of Sciences (PNAS)," this study paves the way for future advancements in HIV-1 treatment.
Publication: https://www.pnas.org/doi/10.1073/pnas.2306374120
An interview with Prof. Gohlke about this research was published on 28.02.2024.
Contact: Prof. Holger Gohlke
