Nanoparticle synthesis
1. Ordered nanoparticles with the micellar technique
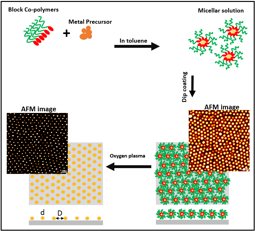
The micellar approach is a bottom-up, self-assembly method utilized for nanofabrication. This technique employs intrinsically chemical or physical forces which operate at the nanoscale to assemble basic units into larger structures. Here, the self-organization of macromolecules allows fabrication of well-ordered arrays of nanoparticles with adjustable sizes and interparticle spacing on a variety of substrates. In this method, reversed micelles are formed by dissolving a block copolymer such as PS-b-P2VP in a non-polar solvent. The reverse micelles provide nanosized compartments for the metal salt precursor as shown in figure below. The interparticle distance can be adjusted by choosing the appropriate PS block length. Using the dip-coating technique, the reverse micelles confining metal salt can be transferred on the surface of the substrate as a self-assembled monolayer. The metal nanoparticles of definite size can be finally formed by oxygen plasma treatment.
2. Disordered nanoparticles with the silanization method

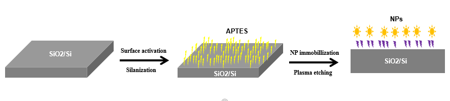
In this method, metal nanoparticles (for example, citrate stabilized NPs) will be immobilized on silicon wafer with different sizes of NPs and controllable NP densities. The silicon wafer is first activated by oxygen plasma and then silanized by 3-Aminopropyltriethoxysilane (APTES) in the glove box under inert condition by vapor deposition to avoid the exposure of the water sensitive silane to air humidity. The stabilized nanoparticles in liquid are then immobilized on the APTES self-assembled layer of silicon wafer through electrostatic adsorption between positive amino groups of APTES and negative citrate shell of NPs. The size of immobilized NPs can be chose by different NP solutions and the densities can be tuned by controlling immobilization times. The unbound negative citrate shell of NPs can be removed by proper oxygen plasma treatment.
Contact:
Dr. Dirk Mayer
Tel.: +49-2461-61-4023
e-mail: dirk.mayer@fz-juelich.de
