Mesoscale modeling
Neuronal signaling is sustained by a complex network of molecular interactions (involving thousands of different partners) highly coordinated over time and space. The molecules involved in the signaling are indeed not homogeneously distributed within the cell and the cell environment is not homogeneous. Signaling initiates within the two-dimensional plane of the post-synaptic neuronal membrane and then move downstream through the three-dimensional volume of the cytosol. The molecular partners interact transiently for executing their specific function in distinct cellular compartments. Knowing how geometrical constraints, partners localization, membrane composition, diffusion, protein-protein interactions, crowding, electric fields, short- and long-range interactions combine together and control signaling transmission over time and space would provide important hints on the understanding and modeling of the signaling. This is an intrinsically multiscale problem involving system sizes and time scales going beyond the capabilities of state-of-the art MD simulations.
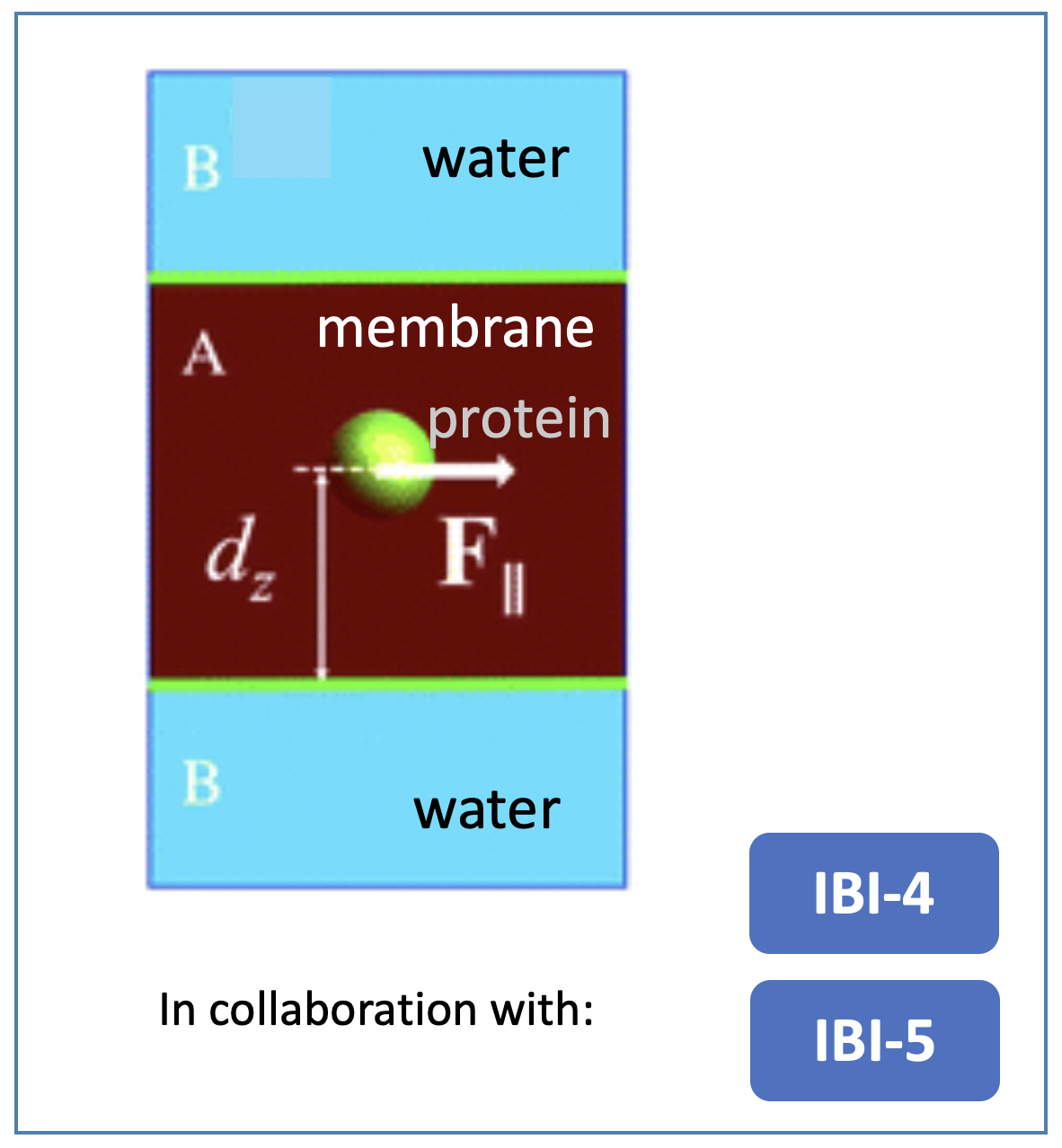
To overcome this limitation while achieving an effective resolution in the modeling of the key physico-chemical features driving the kinetics of the signaling, we develop physics-based mesoscale models. Most of our current efforts focuses on the refinement of stochastic schemes based on Generalized Langevin dynamics and Multiparticle Collision dynamics. The latter is done in collaboration with IBI-4 and IBI-5 Institutes. The developed schemes are specifically conceived to target the events occurring at the membrane (although they can in principle be extended also to other environments). Typically, the parameters used as input to such mesoscopic models are derived following a bottom-up approach by higher resolution molecular dynamics simulations.
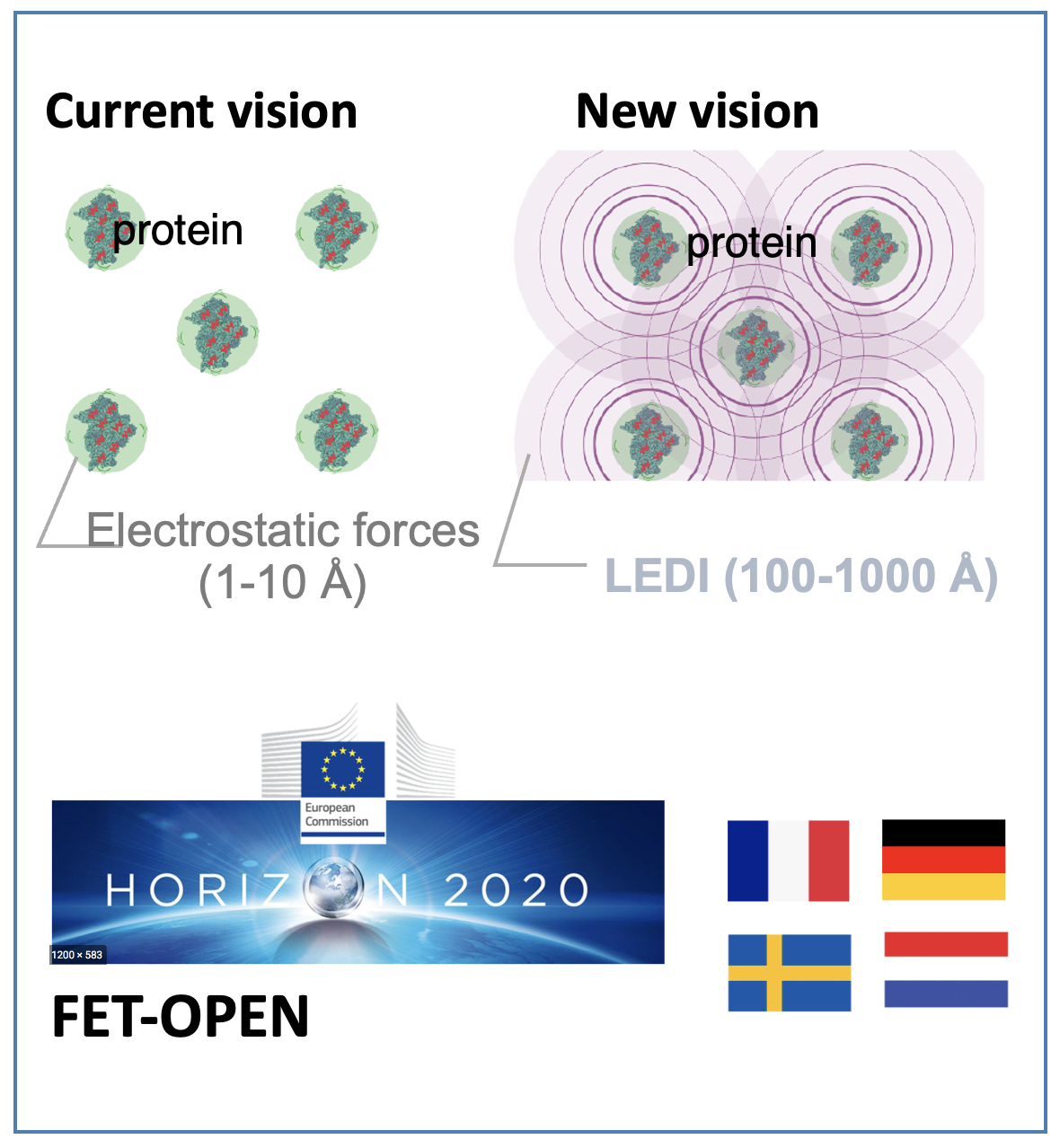
A key aspect of the signaling modeling, is represented by the protein-protein interaction mechanisms. Within the EU project LINkS, recently funded by H2020 FET OPEN program, we focus on the mechanisms possibly leading to the activation of long-range electrodynamic interactions between proteins, which might have a tremendous impact on the signaling.