Flexible intraretinal implants
Development and in vitro validation of flexible intraretinal probes, - Rincón Montes V., Gehlen J., Ingebrandt S., Mokwa W., Walter P., Müller F. and Offenhäusser A., Sci Rep 10, 19836 (2020).
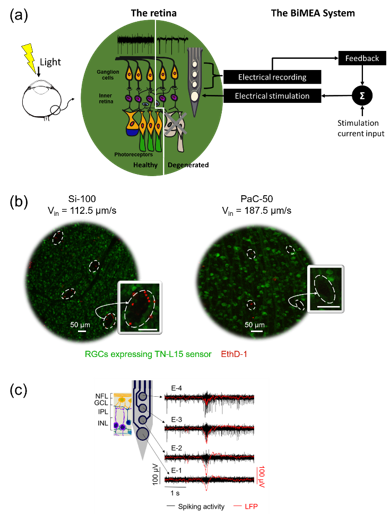
In the last decades, the development of retinal implants has shown meaningful but still rudimentary progress in the restoration of useful vision in blind patients with retinal degenerative diseases due to photoreceptors death. To improve the efficiency of current retinal implants and to understand better the physiology of both healthy and degenerated retinas upon electrical stimulation, a feedback about the retinal activity is desirable. Given the above, our research consortium, BiMEA, has proposed the development of a bidirectional communication strategy in which retinal implants come in close contact with vital neurons in the inner retina to perform electrical stimulation while recording the electrical activity of the retina, as sketched in Figure (a). The door for a bidirectional communication device that stimulates and records intraretinally was demonstrated previously by the recent use of silicon-based penetrating probes (Rincón Montes et al., Front. Neurosci. , 2019). However, the biological impact induced by the insertion of such rigid devices was still unknown.
In this work, Viviana Rincón Montes, in collaboration with co-workers from the Institute of Molecular and Cellular Physiology (IBI-1) of Forschungszentrum Jülich and the Institute of Materials in Electrical Engineering 1 and the Department of Ophthalmology of the RWTH Aachen University, exposed the development and the insertion strategy, as well as the first in vitro application of intraretinal probes based on flexible tissue-like materials in both healthy and degenerated retinas. Furthermore, a systematic study to validate the low acute insertion footprint of flexible penetrating probes compared to the initial silicon devices was performed.
Results showed that probes based on flexible materials, such as polyimide and parylene-C, in combination with a narrow shank design 50 μm wide and 7 μm thick, and the use of insertion speeds as high as 187.5 μm/s will reduce the insertion footprint. Figure (b) shows a comparison of the biggest and smallest acute insertion footprint generated by a silicon and a parylene-C probe, respectively, during intraretinal insertions in TN-L15 mouse retinas. Likewise, the vitality of the tissue and recordings of the neural activity at different intraretinal depths was demonstrated for healthy and degenerated retinas. Figure (c) shows an example of neuronal recordings captured in a degenerated rd10 retina using the flexible BiMEAs, capturing in turn a pathologic activity comprising burst of spikes concomitant with low frequency oscillations, which are present in diseased mouse retinas. Thus, this work opens the door and sets the groundwork for the development of future in vivo intraretinal applications.
Publication: Rincón Montes, V., Gehlen, J., Ingebrandt, S., Mokwa, W., Walter, P., Müller, F., and Offenhäusser, A., Development and in vitro validation of flexible intraretinal probes. Sci Rep 10, 19836 (2020). https://doi.org/10.1038/s41598-020-76582-5
Contact:
Prof. Dr. Andreas Offenhäusser
Institute of Biological Information Processing-Bioelectronics (IBI-3)
Tel.: +49 2461 61-2330
E-Mail: a.offenhaeusser@fz-juelich.de