Functional Collective Dynamics of Domains
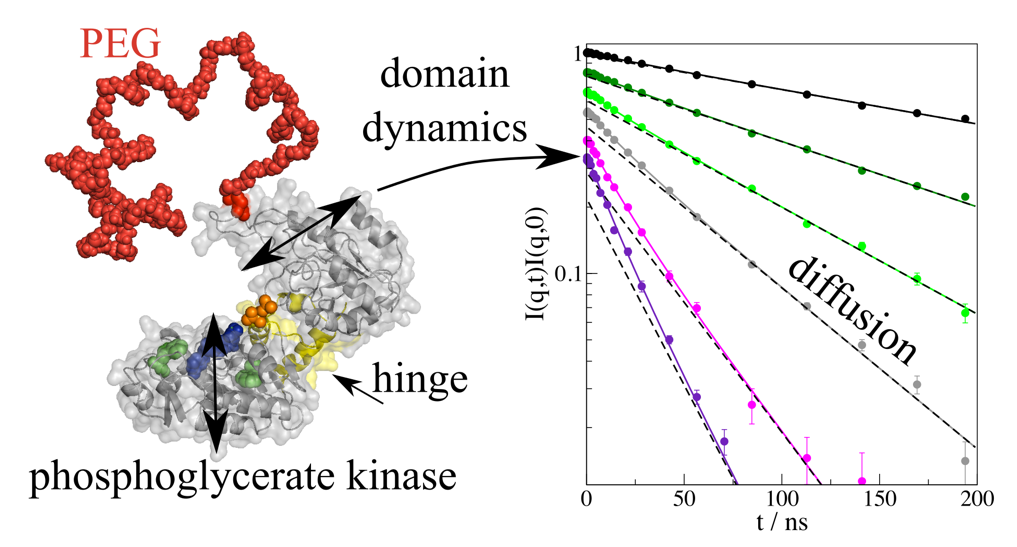
To perform their function structural changes are often important. They reach from atomic reorientation to rearrangements of complete domains to enclose substrates, to release products or to reconfigure domains in complexes. Specific proteins are used as drugs e.g. specific immunoglobulins to target diseases like cancer. Often these proteins are modified by complexation with polymers (e.g. PEG) to improve bioavailability. Our general goal is to describe protein structure and interactions and to identify functional domain motion with their characteristic timescale by combining SAXS/SANS and NSE. In recent studies we observed the domain motions of immunoglobulin IgG1 [1] or the influence of PEGylation onto the functional hinge motion of phosphoglycerate kinase [2].
References:
- L. R. Stingaciu, O. Ivanova, M. Ohl, R. Biehl, and D. Richter,
Fast antibody fragment motion: flexible linkers act as entropic spring
Sci. Rep. 6, 22148 (2016). - K. Ciepluch, A. Radulescu, I. Hoffmann, A. Raba, J. Allgaier, D. Richter, and R. Biehl,
Influence of PEGylation on Domain Dynamics of PhosphoglycerateKinase: PEG Acts Like Entropic Spring for the Protein
Bioconjug. Chem. 29, 1950 (2018).
Contact:
Phone: +49 2461 61-4502
Email: a.stadler@fz-juelich.de


