Electronic and Chemical Properties of Organic Molecules
Over the last years and in collaboration with our colleagues at Karl-Franzens Universtiy in Graz we have developed photoemission tomography into a powerful technique that provides direct access to the orbital structure of molecules (in particular of frontier orbitals that determine the electronic, optical and chemical properties of molecules) and thus helps revealing electronic and chemical states of molecules at surfaces.
Photoemission tomography is a combined experimental and theoretical technique in which the results of angle-resolved ultraviolet photoemission (ARUPS) are interpreted in terms of the orbital structure, in momentum space, of the electron’s initial state in the molecule before it is photoemitted. Most importantly, this allows the determination of the energy alignment of the molecular orbitals with respect to the Fermi level of the substrate [1], even if the orbitals are so closely spaced in energy that they cannot easily be resolved on the energy axis alone [2, 3]. The trick is that the deconvolution is carried out in momentum space, and not on the energy axis. The momentum space information also allows to pinpoint symmetry breaking of the molecule at the surface through the lifting of orbital degeneracies [4]. Because orbital shapes are rigidly fixed to the structure of the molecule, photoemission tomography reveals molecular orientations as well.
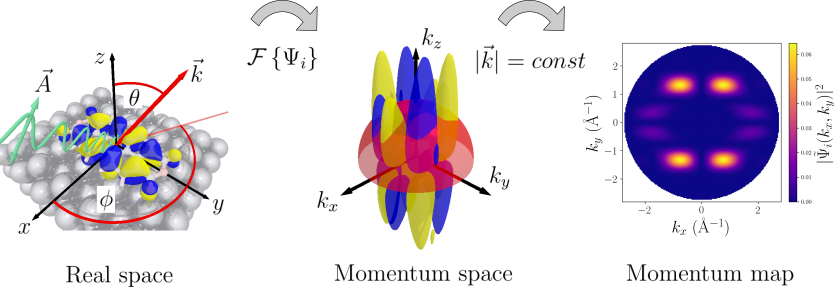
Contrary to initial expectations, we find that photoemission tomography can be applied to strongly interacting molecule/metal interfaces, for example, when molecular orbitals form dispersing bands [5], or to small molecules [6]. This is remarkable, because it shows that the approximation of the final state of the photoemitted electron as a plane wave also works unexpectedly well in these cases.
In our study of charge transfer at molecule/metal interfaces we find that, despite widespread expectations, a thin insulating layer grown on a metal can actually promote charge transfer to the molecule instead of suppressing it [7, 8, 9], because it reduces the work function. Tuning the interaction between molecules and substrate at the interface, one can gain control over the molecular charge state, be it fractional or integer [10], or even create organic films containing multiple charge states, i.e., coexisting neutral and charge molecules, both in multi-compound [11, 12] and unary monolayers [13].
Finally, photoemission tomography is a valuable tool to investigate chemical reactions. For example, we have used momentum space “fingerprints" of frontier orbitals to identify the exact chemical nature of reaction intermediates and products synthesized either ex-situ by “wet chemistry” or directly at the surface [14].

