U. Benjamin Kaupp
Caesar and University of Cologne

Study of cellular signalling in sensory cells and neurons. Biophysics of ion channels and membrane receptors. Chemo-sensation of sperm. Molecular neurobiology. Various spectroscopic techniques to study the dynamics of protein function and folding.
Vita
U. Benjamin Kaupp studied chemistry and completed his dissertation in 1979 at the Max-Volmer-Institut for Biophysical Chemistry of the Technical University Berlin. As a post-doc in the USA, he spent a year at the Department of Physiology & Biophysics at SUNY Stony Brook. In 1987, he worked as a Feodor-Lynen-Fellow of the Alexander-von-Humboldt Foundation at the Department of Medical Chemistry in Kyoto. From 1988 to 2007 Kaupp was Director at the Institute of Neuroscience and Biophysics, Forschungszentrum Jülich. Since 1988 he is Professor of Biophysical Chemistry at the University of Cologne. Since 2008 Kaupp is Professor of Molecular Neurobiology at the University of Bonn and Scientific Member of the Max Planck Society. In 2008 Kaupp became Scientific Director of the Center of Advanced European Studies and Research (caesar) Bonn, an associate of the Max Planck Society.
Contact: u.b.kaupp@caesar.de
Molecular Sensory Systems
Download this page as a PDF file (PDF, 358 kB)
The department of Molecular Sensory Systems studies signaling in cells. We want to understand how cells register a stimulus and transform the stimulus into a cellular response. This is a fascinating question that occupies many scientists worldwide. A profound understanding of the complex signaling events inside cells requires modern biological, chemical, and physical techniques. Therefore, biologists, chemists and physicists work closely together in our department.
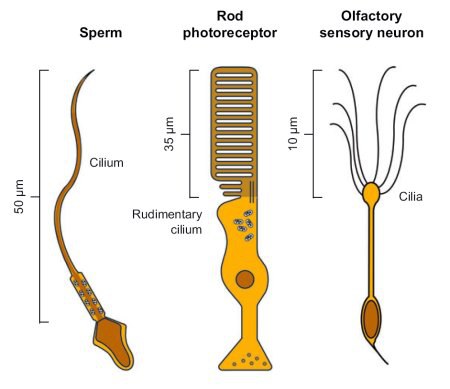
Figure 1: Three examples of sensory cells. Scheme of a sperm, a rod photoreceptor and an olfactory sensory neuron (OSN).
Processing of sensory signals
Sensory cells transform a stimulus into an electrical signal. For example, photoreceptors in the retina of the eye detect light and generate an electrical signal via a cascade of biochemical reactions. This signal is then relayed to brain areas that compose the myriads of information into a visual scene. Likewise, olfactory sensory neurons react to odorants with an electrical signal that encodes the quality and intensity of the odor. We aim at elucidating the molecular mechanisms underlying sensory signaling. To this end, we investigate the structure, function, and interactions of proteins that are involved in the generation of the cellular signal. In particular, we study ion channels that produce the electrical excitation. Among these ion channels are the cyclic nucleotide-gated (CNG) channels and the pacemaker (HCN) channels.
Chemosensation in sperm
For successful fertilization, motile sperm need to find the immotile egg. Sperm are propelled by a flagellum; for orientation, they use chemical factors (chemoattractants) that are released by the egg. The chemoattractants are detected by specific receptors on the sperm surface. Surprisingly, sperm employ similar mechanisms and signaling molecules as photoreceptors and olfactory neurons. Sperm display an astounding sensitivity: they can register and respond to a single molecule of the chemoattractant. We study chemosensation in sperm of sea urchins and humans and identify the receptors, cellular messengers, and ion channels that endow sperm with this spectacular sensitivity.
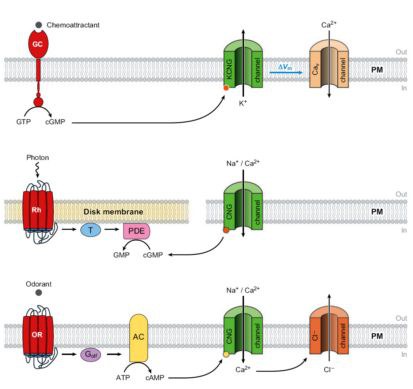
Figure 2: Signaling in sperm, photoreceptors, and olfactory sensory neurons (OSNs). Simplified models of the signaling pathways in sperm from marine invertebrates (top), vertebrate rod photoreceptors (middle), and OSNs (bottom). AC, adenylyl cyclase; CNG, cyclic nucleotide gated; GC, guanylyl cyclase; Golf, olfactory-specific G protein; OR, odorant receptor; PDE, phosphodiesterase; Rh, rhodopsin; T, transducin; PM, plasma membrane.
Optical methods
In collaboration with the Leibniz-Institute for Molecular Pharmacology in Berlin, we develop optical switches that are employed for the photonic control of receptors and ion channels. In fact, these compounds are "Trojan horses" that are smuggled into the cell. Once inside, the signal molecules are released upon exposure to light. With the help of these Trojan horses, the signal transduction can be followed with high spatial and temporal resolution.
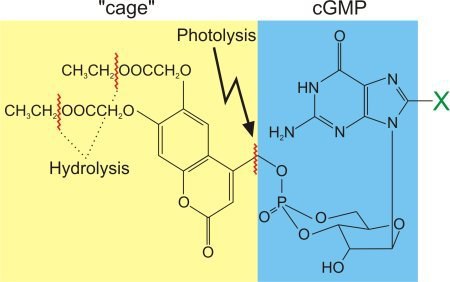
Figure 3: Caged cGMP - a Trojan horse. The uncharged ester side chains of the "cage" are hydrolyzed once the compound arrives inside the cell. The now charged compound cannot leave the cell anymore: it is trapped inside the cell. However, due to its "cage", cGMP is invisible to the cell. Only upon irradiation with a short flash of UV light (photolysis), the molecule is released in its free, active form. X denotes a potential substitution site: for example, 8-Br-cGMP is a hydrolysis-resistant derivative of higher biological efficacy than cGMP.
