Microbial synthesis of aromatic (plant) natural products and other aromatic molecules of biotechnological interest
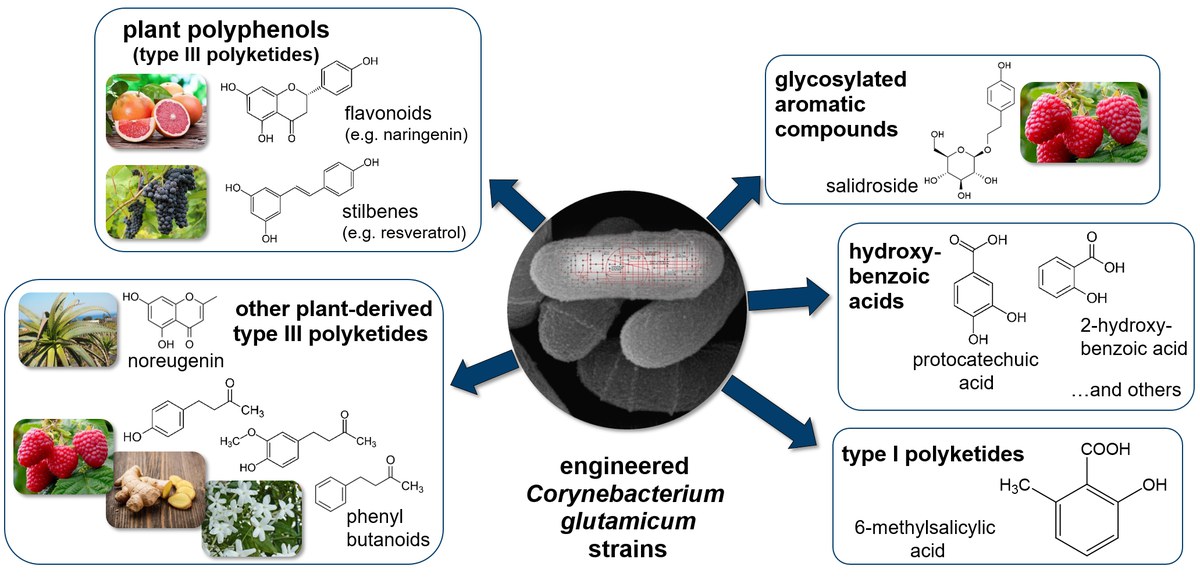
Natural products are metabolites of the secondary metabolism that are not directly involved in growth, development or reproduction, but have important functions in defense and signaling, or serve as pigments or fragrances. Of the many hundreds of thousands natural products known to date, many demonstrate important pharmacological activities or are of biotechnological significance. However, isolation from natural sources is usually limited by low abundance in the producing organisms, whereas total chemical synthesis is typically commercially unfeasible considering the complex structures of most natural products. With advances in DNA sequencing and recombinant DNA technology, many of the biosynthetic pathways responsible for the production of these valuable compounds have been elucidated, offering the opportunity of a functional integration of biosynthetic pathways originating from plants or other microorganisms in the production strain of choice [1,2].
We apply latest molecular tools in the high-throughput format for the construction of tailor-made recombinant Corynebacterium glutamicum strains by metabolic engineering to modify metabolic profiles according to individual production purposes. This includes the heterologous expression of metabolic pathways to confer the capability for natural product synthesis, but also host cell engineering for optimized substrate utilization, improved precursor supply, reduced product degradation or product secretion [3]. Furthermore, we develop cultivation and in situ extractive strategies to increase product formation and minimize undesired product oxidation [4]
This approach offers the promise to provide sufficient quantities of the desired natural products from inexpensive and renewable resources [5,6].
References:
[1] Milke L. and Marienhagen J. (2020). Engineering intracellular malonyl-CoA availability in microbial hosts and its impact on polyketide and fatty acid synthesis. Appl. Microbiol. Biotechnol. 104: 6057-6065. (https://doi.org/10.1007/s00253-020-10643-7)
[2] Kallscheuer N., Classen T., Drepper T., Marienhagen J. (2019). Production of plant metabolites with applications in the food industry using engineered microorganisms. Curr. Opin. Biotechnol. 56: 7–17. (https://doi.org/10.1016/j.copbio.2018.07.008)
[3] Mutz M., Brüning V., Brüsseler C., Müller M.-F., Noack S., Marienhagen J. (2024). Metabolic engineering of Corynebacterium glutamicum for the production of anthranilate from glucose and xylose. Microb. Biotechnol. 17: e14388. (https://doi.org/10.1111/1751-7915.14388)
[4] Tharmasothirajan A., Wellfonder M., Marienhagen J. (2021). Microbial polyphenol production in a biphasic process. ACS Sustain. Chem. Eng. 9: 17266–17275. (https://doi.org/10.1021/acssuschemeng.1c05865)
[5] Milke L., Mutz M., Marienhagen J. (2020). Synthesis of the character impact compound raspberry ketone and additional flavoring phenylbutanoids of biotechnological interest with Corynebacterium glutamicum. Microb. Cell Fact. 19: 92. (https://doi.org/10.1186/s12934-020-01351-y)
[6] Tharmasothirajan A., Melcr J., Linney J., Gensch T., Krumbach K., Ernst K.M., Brasnett C., Poggi P., Pitt A.R., Goddard A.D., Chatgilialoglu A., Marrink S.W., Marienhagen J. (2023). Membrane manipulation by free fatty acids improves microbial plant polyphenol synthesis. Nat. Commun. 14: 5619. (https://doi.org/10.1038/s41467-023-40947-x)
