The driving power
Hydrogen stimulates great expectations. It is expected to ensure the energy transition. At the same time, hydrogen technologies are intended to become a new export hit for Germany. The potential is there, according to Jülich researchers. They are addressing all aspects regarding this beacon of hope.
Beacon of hope
Hydrogen is the smallest and lightest chemical molecule. Still, it plays an important role in the restructuring of the energy system.
Germany is to become a global pioneer in hydrogen. This is the intention of the Federal Government’s National Hydrogen Strategy. Hydrogen is not only considered a central element for Germany to achieve its climate targets by 2050; it is also regarded as the urgently needed building block for networking and optimising electricity, transport, industry and the heating supply. Germany is to secure a leading international position in hydrogen technologies, thus opening up new sales markets for the German economy. Following the example of space travel, Federal Minister of Education and Research Anja Karliczek even speaks of establishing a “Cape Canaveral of hydrogen” in Germany.
Hydrogen is attractive because it can be used to store electricity from renewable energy sources, as green electricity is generated very discontinuously: sometimes the wind blows forcefully, sometimes not at all. The sun does not always shine with the same intensity, either. Surplus electricity that is not immediately needed in the grid could be used to produce hydrogen. “This can be stored over long periods of time and then be used when there is no wind, for example,” says Prof. Olivier Guillon from Jülich’s Institute of Energy and Climate Research (IEK-1).
However, it is not only this storage function that makes hydrogen essential for the energy transition. It also offers a way out of a dilemma: after all, it is not foreseeable that all aircraft, ships and trucks can ever be powered electrically by batteries. “Even so, in the transport sector we have to abandon the classic supply of diesel or petrol if we want to emit only the same amount of greenhouse gases into the atmosphere in Germany in 2050 as we take from it,” says Prof. Detlef Stolten, who works on energy systems at IEK-3. The solution could be fuel cells – climate-friendly drives that use “green” hydrogen.
But that’s not all: hydrogen can also help the chemical industry through a difficult transition, as it is dependent on carbon sources to produce medicines and plastics, for instance. As long as it relies on mineral oil or natural gas for this purpose, the result will be a poor climate balance. “Using so-called Power-to-X technologies, hydrogen and, if CO2 is added, carbonaceous gases can be produced from green electricity. These could replace mineral oil and natural gas to produce basic chemicals for the industry and liquid fuels, such as for aviation. In this way, hydrogen links the sectors of electricity, industry and transport,” emphasises Prof. Rüdiger Eichel, electrochemistry expert at IEK-9.
However, there are still some obstacles to overcome: a lot of energy is lost in the production, storage and use of hydrogen. This drives up costs. The infrastructure for transporting and refuelling hydrogen safely is also expensive. In addition, there is hardly any operating experience with some of the hydrogen technologies so far.
Jülich energy researchers are working on paving the way for hydrogen. “We have comprehensive, holistic expertise in this: it ranges from basic research to application – starting with the materials and electrochemistry and leading on into the key technologies and understanding of systems, which allows us to make technical, societal and economic assessments,” says Olivier Guillon. According to the materials scientist, this is unique in Germany. So, these are the best prerequisites for hydrogen to really fulfil its role as a beacon of hope.
The producers
Electrolysis systems produce the hydrogen, Jülich researchers improve them.
The windmills are turning fast. Production of green electricity is running at full speed. Electricity, not needed by the computers in offices, households and industry at the given moment, can be used to split water in electrolysis systems. In this way, hydrogen is produced and the electrical energy is converted into chemical energy. This allows excess electricity to be stored.
“We are working on the optimisation of three different electrolysis processes,” says Dr. Martin Müller, process engineering specialist at the Institute of Energy and Climate Research (IEK-14). “Each process has its strengths and weaknesses. It is still open which one will win the race, and it also depends on whether the electrolysis system is installed directly at a wind farm, a home solar power system or in a chemical network, for example.”
Type 1: The classical one
Plants where the central component, the electrolyte, is an alkaline liquid can be bought off the shelf, so to speak. Alkaline electrolysis plants make do with inexpensive materials. A major disadvantage is their low power density: they produce comparatively little hydrogen per square centimetre of surface area. Large space requirement and high material consumption are the result. “Alkaline electrolysers are generally considered to be technically mature, but we are pursuing new approaches to increase their power density,” says Müller. One of these approaches is based on new partitions which are installed in the liquid electrolyte to electrically isolate the negative pole (cathode) and the positive pole (anode) from each other.
Type 2: The promising one
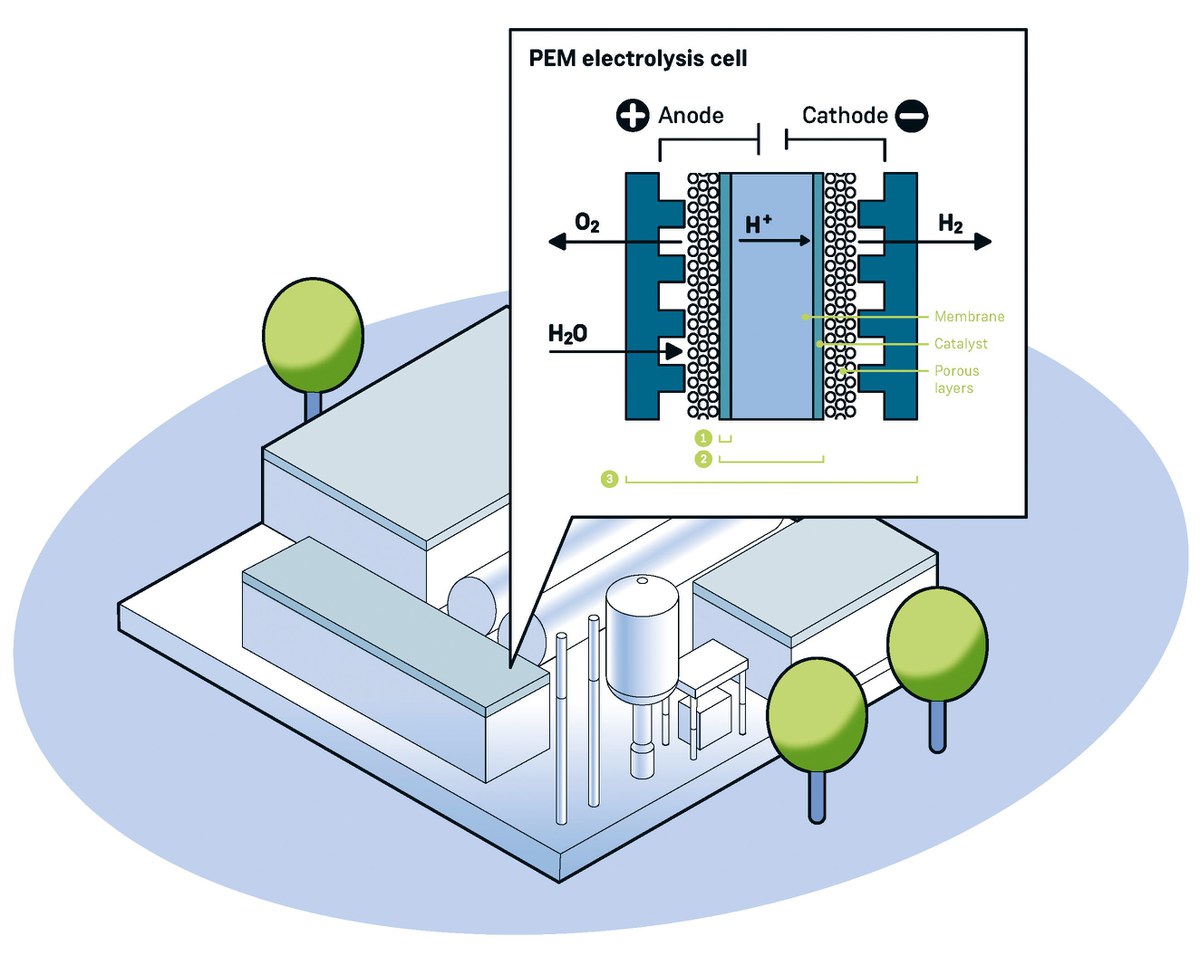
Higher power densities than alkaline electrolysers are achieved in systems in which the electrolyte does not con-sist of a liquid but of an extremely thin layer, a so-called polymer electrolyte membrane (PEM). However, the high costs of PEM plants stand in the way of wider dissemination. “Jülich researchers have their sights set on various parts of the facility to change this,” says Dr. Marcelo Carmo, electrochemist at IEK-14.
Where Jülich researchers improve the cell
- PEM systems require expensive and rare noble metals such as platinum and iridium. Yet, Jülich researchers have recently developed an anode (positive pole) which needs no more than a touch of iridium oxide to achieve excellent results. With such electrodes, PEM systems could be realised that only require about 10 per cent of the iridium quantity used so far.
- Jülich scientists have produced the unit, which consists of a coated membrane and electrodes, using a process that is suitable for mass production and yet flexible: the substances for the individual layers are each finely distributed in liquids and the resulting dispersions are applied step by step with a slotted nozzle.
- A new design of PEM electrolysis systems with very thin membranes makes it possible to supply the water in a different way than conventionally. According to calculations, this results in a 15 per cent reduction in investment costs.
Type 3: The hot one
While alkaline and PEM electrolysis systems are usually operated at around 80 °C, the third electrolysis process requires more than 650 °C. In these so-called SOE systems (Solid Oxide Electrolysis), the high operating temperature must be maintained even when no current is available. This is because starting and stopping the system would cost even more energy – and this would also tire out the material more quickly. The technology is economically interesting nevertheless: “The SOE systems are very well suited to use the heat generated by many industrial processes. Then, they convert the electricity very efficiently into the chemical energy of hydrogen,” explains Prof. Ludger Blum of IEK-14. In recent years, Jülich researchers have made SOE electrolysers more reliable and durable through various improvements.
The liquid carrier
A process developed by the Helmholtz Institute Erlangen-Nürnberg makes it possible to store and transport hydrogen safely and easily. By 2022, it will be tested in everyday operation at Jülich.
Hydrogen leaving the electolyser is a colourless and odourless gas, lighter than air. But what to do with it? It must be stored and transported before it can be used at a later date or in another place. Lest it take up too much space, it is usually compressed and stored in pressure vessels, underground in salt caverns or cooled to below minus 240 °C so that the hydrogen becomes liquid. There is an alternative, however: the so-called LOHC technology. In a chemical reactor, hydrogen is bound to a diesel-like and flame-retardant organic carrier liquid, the Liquid Organic Hydrogen Carrier, or LOHC for short.

In the same reactor, the hydrogen can be split off again as soon as it is later needed for power generation or for fuelling fuel cell vehicles. Bonded to the LOHC, the hydrogen can not only be safely stored at atmospheric conditions in classic steel tanks, but can also be transported in classic tank trucks, tank wagons or tank ships. The technology is based on research work carried out by a team led by Prof. Peter Wasserscheid at the Helmholtz Institute Erlangen-Nürnberg, a part of Forschungszentrum Jülich.
At the Jülich research site, an LOHC facility that is unique in the world will be tested in daily operation by 2022. It will become part of the “Living Lab Energy Campus”, a real laboratory for future energy systems at the Jülich research campus. The LOHC facility will be coupled to a combined heat and power plant and use the waste heat generated there to release the hydrogen from the carrier liquid. The hydrogen storage process, in turn, releases heat which flows into Forschungszentrum Jülich’s local heating network.
The sources of power
Thanks to Jülich research, fuel cells are becoming more cost-effective and more efficient.
The hydrogen flows through pipelines to combined heat and power plants and filling stations. LOHC trucks supply small places that are not connected to the pipeline systems. Fuel cells are used to release the maximum of the stored energy. They generate electricity from the hydrogen. For this purpose, they additionally require oxygen; the only exhaust produced is water. In the combined heat and power plants, the fuel cells generate electricity for housing estates, also using the heat produced in the process. This increases efficiency. In vehicles, fuel cells drive engines.
So-called SOFC fuel cells are particularly well suited for combined heat and power plants (CHP). SOFC stands for Solid Oxide Fuel Cell. This type is extremely efficient in converting hydrogen into electricity. Since SOFC fuel cells in CHPs can run continuously, there is no need to frequently start up the system to the required operating temperature of around 700 °C, which would cost energy and put a strain on materials. For fuel cells to be operated economically, they must last as long as possible. With a SOFC fuel cell they developed themselves, Jülich researchers have proven that cells of this type function perfectly for more than ten years in continuous operation. “At the beginning, hardly anyone would have thought so because of the high operating temperature and the resulting material requirements,” says Prof. Ludger Blum from IEK-14.

In addition, Jülich researchers have developed a solid oxide system on a laboratory scale that they can switch back and forth within ten minutes: between an electrolysis mode, in which it produces hydrogen with electricity, and a fuel cell mode, in which it generates electricity from hydrogen. “If a plant can be operated as an electrolyser or as a fuel cell, depending on requirements, then instead of two plants only one plant is needed to store electricity in the form of hydrogen on site and convert it back into electricity at a later date. This helps to save considerable costs,” explains Blum. Besides, such reversible cells are suitable for use in remote stations on islands or mountains. The performance of the reversible cell still decreases quite rapidly with increasing operating time, especially in electrolysis mode, but the researchers are working to change that. They are also in the process of transferring their findings from the laboratory scale to larger plants.
Another Jülich team, together with researchers from TU Wien (Vienna), has increased the power density of so-called metal-supported SOFCs by more than 200 per cent within a few years. “The decisive factor was that we systematically optimised the structure of the electrochemical functional layers and the cell architecture,” says Dr. Martin Bram of IEK-1. Car manufacturers are interested in metal-supported SOFCs, which they would like to use in electric cars as range extenders in order to continuously charge the vehicle battery. Metal-supported SOFCs are particularly suitable for this purpose because they can withstand shocks and vibrations on the vehicle floor better than the usual full ceramic fuel cells.
The value adders
Jülich researchers are working on sustainable processes to produce basic chemicals and liquid fuels using green hydrogen.
Production is rolling: synthetic materials, varnishes, adhesives, medicines and fuels are produced in the factories of the chemical park. The elementary components of these products – hydrogen, oxygen and carbon – have so far mostly come from crude oil and natural gas. “Power-to-X technologies can change that. Here, power stands for sustainable electricity, X for added value,” explains Jülich energy researcher Prof. Rüdiger Eichel. The elements are then supplied by water and carbon dioxide (CO2), which is separated from industrial waste gases or the atmosphere. Electricity from wind and sun provides the energy needed to rearrange the elements, so to speak, and to turn them into fuels or basic chemicals. Thus, the production process does not generate any climate-damaging gases.
Eichel coordinates the “Kopernikus project P2X” in which 49 partners from industry, science and civil society are jointly researching and developing Power-to-X technologies. The researchers were particularly successful regarding the starting point of the process chain: the so-called co-electrolysis units. These not only use electricity to split water, but also convert CO2, producing a mixture of carbon monoxide and hydrogen. Experts call it syngas because it can be used for synthesis, i.e. for the production of various chemicals.

As a special feature, the electrolysis system developed by Jülich researchers in the P2X project can produce syngas in which the mixing ratio of hydrogen and carbon monoxide can be set as desired. “This is crucial to ensure that the syngas has the right composition for the desired fuel or required basic chemical,” says Eichel. The P2X researchers are also developing the facilities with which the syngas can then be processed into fuels such as synthetic diesel or kerosene. “In this way, the supply of liquid fuel for high-performance vehicles such as aircraft, ships and trucks is to be made renewable in the future,” says Prof. Ralf Peters of IEK-14. He coordinates the fuel synthesis activities in the C3-Mobility research association in which 30 partners from science and industry – including many car manufacturers – research climate-neutral fuels for the traffic of the future.
“Together with our partners from science and industry, we want to bring the technologies that we develop in the various projects into widespread application as quickly as possible. The structural change in the Rhineland’s lignite mining area offers great opportunities in this respect,” says Eichel. This is the aim of the iNEW project (Inkubator für Nachhaltige Elektrochemische Wertschöpfung; incubator for sustainable electrochemical added value), which the German government is funding with over € 20 million as part of its immediate action programme for structural change. “Previously, energy-intensive industries have always been located in regions where coal, gas or oil was extracted. Now we have the opportunity to put this alliance of energy and added value on a sustainable track,” says project manager Eichel.
Text: Frank Frick | Illustrations/Images: Forschungszentrum Jülich/SeitenPlan/Bernd Struckmeyer