Oxygen atoms made directly visible in the electron microscope
Sharp insights into ceramics and superconductors
[7. Februar 2003]
Scientists at Research Centre Jülich have made individual oxygen atoms directly visible with an electron microscope in a certain class of materials, the perovskites. Their recipe for success: they have developed a technique to correct the unavoidable aberrations in the microscope. In commercial instruments, these aberrations inevitably lead to blurred images in which no individual oxygen atoms can be recognized. The Jülich scientists' findings have been published in the latest issue of the prestigious journal "Science" (Science, 7th February 2003).
Ceramic materials on the basis of oxides with perovskite structure - including barium and strontium titanate - play a major role in modern electronics. Their application is already widespread, for example as chips in phonecards or pay-cards. Perovskites are also the base material for high-temperature superconductors and will be increasingly needed in future microelectronics where they will be used in ultrathin films of just a few tens to several hundreds of atomic layers. One of the most important problems on the way to this goal is the correct adjustment of the oxygen content of these oxides, which then has to be maintained throughout the large number of process steps in device fabrication. "The oxygen content critically determines the electrical properties of the perovskite oxides", Prof. Knut Urban from the Jülich Institute of Solid State Research explains the problem. "Since even the absence of a few oxygen atoms in the electrically activezones of the thin films would seriously impair their function they must be fabricated with almost atomic precision."
Transmission electron microscopy can be used in principle to check whether this atomic precision has actually been achieved. Researchers have therefore been attempting to make the oxygen atoms directly visible in the microscope since the end of the eighties - so far without success. The basic principle of electron microscopy is quite simple: An electron beam penetrates a thin specimen. The outgoing electrons are guided by an electromagnetic lens system, which combines them into a greatly magnified image. However, for various reasons distorted images result in which no individual oxygen atoms can be recognized.
The group headed by Knut Urban has now achieved a breakthrough. The scientists work with the only so-called "aberration-corrected" transmission electron microscope in the world so far. The problem of "spherical aberration" occurs in both optical and electron microscopes. Light or electron beams that pass through the lenses of the microscope close to the edge are deflected too strongly - the image is blurred. With specially shaped magnetic lenses, however, the researchers are able to correct this previously unavoidable aberration. They have - metaphorically speaking - prescribed spectacles for the electron microscope thus focusing its sight. This step has now proved to be extremely successful. It does not only permit oxygen to be imaged atomically for the first time, but also enables the oxygen content to be measured quantitatively in atomic dimensions. Prof. Knut Urban and his co-workers, the microscopy specialist Dr Markus Lentzen and the materialsscientist Dr Chun Lin Jia, have published their groundbreaking findings in the latest issue of "Science".
The scientists from Research Centre Jülich developed the novel correction technique in the nineties together with colleagues from the European Molecular Biology Laboratory (EMBL) in Heidelberg and Darmstadt University of Technology - thus creating the prototype of a completely new generation of microscopes. In the course of this year, the first commercial aberration-corrected electron microscopes will be available worldwide. Knut Urban is convinced that "this method will replace the classical type of high-resolution electron microscopy in many fields of materials science".
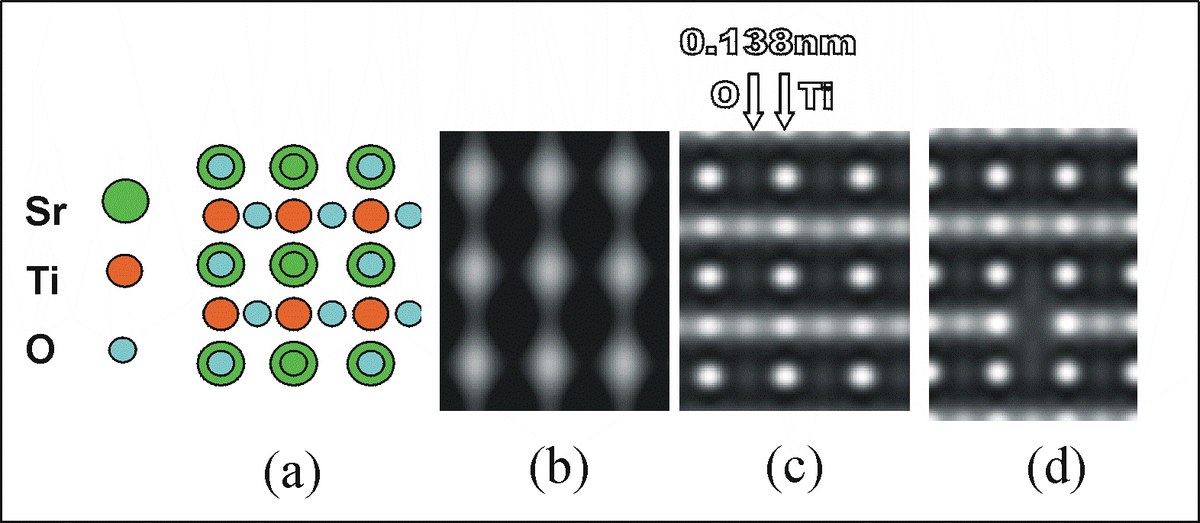
Strontium titanate (chemical formula SrTiO3) in the electron microscope. The structure (a, Sr = strontium, Ti = titanium, O = oxygen) is imaged with a "classical" electron microscope (b) and in comparison with the corrected microscope (c) in a simulation. Whereas previously only the heavy strontium atoms were discernible (b), the lighter oxygen atoms are now also directly visible (c). The scientists can also identify oxygen vacancies (d).
Diagram: Research Centre Jülich

A silver-coloured section in the lower third of the electron microscope reveals the prototype with the corrected optics.
Photograph: Research Centre Jülich
Information:
Dr Renée Dillinger
Science Journalist
Forschungszentrum Jülich
52425 Jülich, Germany
Tel. ++ 49 2461 61-4771, Fax ++49 2461 61-4666
E-mail: r.dillinger@fz-juelich.de
Mechthild Hexamer
Head of Public Relations, Press Officer
Tel. ++49 461 61-4661, Fax ++49 2461 61-4666
E-Mail: m.hexamer@fz-juelich.de
