Alzheimer´s disease – pieces of the puzzle fall into place
Devastating, shattering, frightening – words can barely express the emotions that a diagnosis of Alzheimer’s disease can arouse in those affected and their relatives. Impressive film adaptations of books like ‘Still Alice – My Life Without Yesterday’ and intense narratives like 'Langsames Entschwinden' ('Vanishing Slowly', by German author Inge Jens) have made it easier to talk openly about dementia, seek help and apply for care. This, however, does not change the inevitable feeling of hopelessness experienced upon receiving such a diagnosis.
Fleeting hopes of early treatment with vaccines and antibodies, preventative lifestyle choices like taking up sport, or diets including the likes of coffee and cinnamon are regularly dashed in the news media when yet another major pharmaceutical company cancels its Alzheimer’s research or withdraws from promising studies. This happened in 2016 with the company Eli Lilly, which had invested three billion dollars during 27 years of research and development, Pfizer in January 2018 and Merck in February 2018.
Undeterred by the setbacks of the withdrawal of pharmaceutical companies from the search for a treatment for Alzheimer’s, researchers are working with unflagging persistence towards understanding the molecular basis of this disease and exploring new treatments. Scientists and medical experts at the Forschungszentrum Jülich, for instance, are working on decoding the protein structures involved in the disease, on treatment options and on early diagnosis.
Research into Alzheimer’s dementia is so complex that it resembles an enormous jigsaw puzzle. What the neurologist Aloïs Alzheimer saw through his microscope around 110 years ago turned out to be the first of countless tiny puzzle pieces that make up an unclear picture. The round and elongated protein deposits that he discovered in the brain of his deceased patient Auguste Deter, together with massive cell loss, were merely the striking side effects of a destructive process that had been taking place gradually and invisibly over decades – completely undetectable with a conventional microscope.
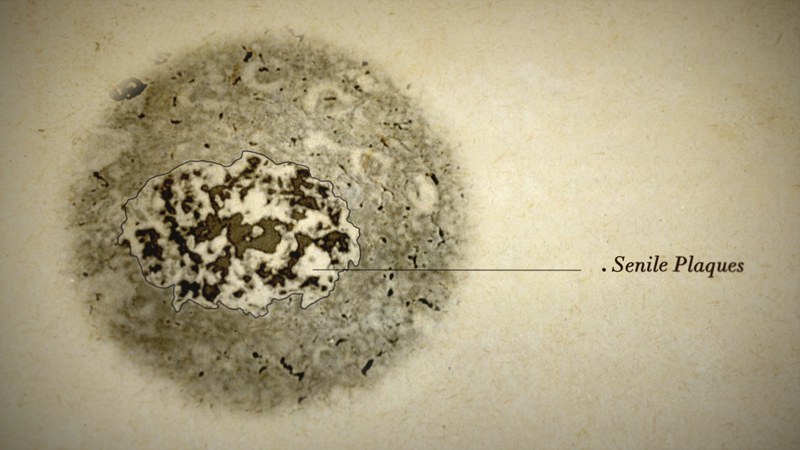
Fatal chains
Only with the advent of technological advances in physics, chemistry and biology have researchers all over the world started to slowly piece together the functions and sites of action of the smaller puzzle pieces in the finely tuned brain metabolism. They recognise which pieces do not fit into the picture and thus cause illness. The 'amyloid hypothesis' for Alzheimer’s dementia currently prevails in the scientific community. What Alzheimer described as 'senile plaques', which accumulate around nerve cells, have been identified as aggregations of special proteins known as amyloid beta proteins. The 'neurofibrillary tangles' that he discovered clustered within the nerve cells consist predominantly of altered tau proteins. These are proteins that normally support healthy neural pathways within nerve cells.
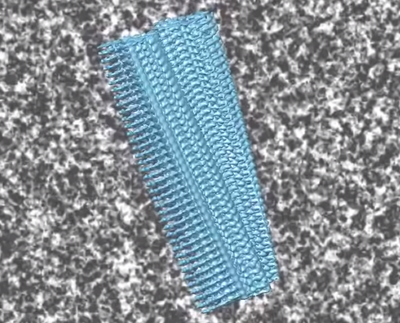
Recent studies suggest that amyloid deposits start forming in the brains of those affected by Alzheimer’s up to 20 years before the first symptoms become apparent. A key finding for both researchers and patients is that not all those with amyloid plaques in the brain go on to develop dementia. Only the accumulation of tau proteins heralds the irreversible mass death of nerve cells – and it is only at this point that the first mental limitations occur.
So why do most common therapies focus on the amyloid cascade hypothesis, while tau proteins receive little attention? Scientists compare amyloid beta with the trigger of a firearm, and tau with the bullet. In order to understand the full picture, it helps to first obtain an understanding of how amyloid beta forms in the body. Throughout life, this molecule is ‘snipped’ from a larger protein, each cell’s own amyloid precursor protein (APP), with the help of two enzymes. Mutations in this protein can have drastic consequences, producing amyloid beta variants with altered properties.
The need for a combined approach
While one mutation leads to the early onset of Alzheimer’s in the patient’s life, a small section of the Icelandic population seems to be almost resistant to the disease due to another mutation in the APP. Therefore, the great majority of drug candidates concentrate either on preventing amyloid beta proteins from forming altogether – by blocking the enzymes involved – or on destroying or neutralising the amyloid beta molecules. Researchers fear that if tau fibrils appear, the disease is probably already too advanced to be halted. However, recent findings indicate that a combined therapy that targets both amyloid beta and certain tau variants is more promising than concentrating on one molecule alone.
In their work, researchers at Forschungszentrum Jülich are initially targeting amyloid beta, especially its aggregates. The drug candidate PRI-002 has been developed at the Forschungszentrum and the Heinrich Heine University Düsseldorf. This is able to neutralise toxic accumulations of amyloid beta, known as ‘oligomers’. PRI-002 has resulted in an improvement in cognitive performance in mice with Alzheimer-like symptoms. In September 2017, the Forschungszentrum Jülich set up the company Priavoid GmbH to develop the drug to commercial viability. After successful pre-clinical safety and toxicity testing, the Phase I clinical trial began in April 2018 to test the Alzheimer’s drug safety for humans. Patience is still required – taking a drug from Phase I stage to market approval takes an average of seven years.
Risk increases with age
While the inherited form of Alzheimer’s dementia is very rare, the greatest risk factor is age. Amyloid beta proteins are normally harmless as single molecules. Indeed, they are even credited with having a protective function in the nervous system. With age, however, there is an ever greater likelihood of amyloid beta proteins beginning to agglomerate and form soluble – and extremely toxic – aggregates, known as amyloid beta oligomers. They are considered to be important drivers of the occurrence of Alzheimer’s disease.
It is suspected that these oligomers possess prion-like properties. This means that they can transfer their misfolding behaviour to other amyloid beta proteins. In addition, they can affect normally folded amyloid beta molecules and, ultimately, tau proteins. This creates a snowball effect that causes more amyloid beta and tau oligomers. Unfortunately, tau oligomers also appear to have prion-like properties, setting off other chain reactions of pathological misfolding among tau proteins. This confirms the suspicion that previous approaches to treatment that exclusively targeted the reduction of amyloid beta proteins were ineffective because the fateful chain reaction had long since begun. All of these scenarios culminate in mass cell death.
Small cause, big effect
Even small changes to the blueprint of the amyloid beta molecule can have devastating effects, as structural biologists at Jülich recently demonstrated in an international research project. They were investigating a particularly aggressive variant of the protein – ‘pyroglutamate amyloid beta’, which agglutinates at an extremely high rate, is resistant to degradation processes and forms aggregates that are extremely toxic to brain cells. Pyroglutamate amyloid beta lacks only two amino acids at one end of the molecule, while a third is converted from a glutamate into a pyroglutamate. Although these changes are relatively minor, they alter the biochemical properties dramatically. This pyroglutamate variant agglutinates to form harmful aggregates over 100 times faster than the ‘unshortened’ version of amyloid beta. Therefore, this form of the protein is an important target for potential drugs. The drug candidate PRI-002 developed at Forschungszentrum Jülich can also neutralise the aggregates of this amyloid beta variant.
Securing a diagnosis

Researchers and industry are desperately searching for a reliable and, above all, early diagnostic technique for Alzheimer’s to be able to intervene in the disease process at an early stage. Ideally, an inexpensive blood test would reveal the slightest changes to the cerebral metabolism before too many of the nerve cells are irretrievably lost. Researchers at Forschungszentrum Jülich are targeting amyloid beta oligomers in the hope of establishing this kind of diagnostic technique. Using fluorescent probes, they have been able to detect even tiny amounts of harmful protein aggregates in the spinal fluid of Alzheimer’s patients. In early studies, it was surprisingly easy to distinguish between samples from Alzheimer’s patients and those from healthy control subjects.
In March 2018, on the basis of these scientific successes, a company called attyloid GmbH was founded as a spin-off from the Forschungszentrum Jülich and the Heinrich Heine University Düsseldorf. Since then, the technique has become fully automated and has been extended to the detection of other protein aggregates, such as those found in people suffering from Parkinson’s disease. The next objective is to use blood samples to obtain reliable early diagnosis of neurodegenerative diseases. Other research teams are working towards this same goal. In February 2018 an Australian-Japanese research team presented a blood test that detects whether a patient is showing early signs of Alzheimer’s disease with 90 percent accuracy. Both teams are working hard achieve 100 percent accuracy. Every piece of the puzzle can help find an early treatment and possible cure.
Brigitte Stahl-Busse
