High flyer
They are considered to be promising candidates for the solar modules of the future: materials with a perovskite structure. Prof. Michael Saliba has been heavily involved in this young field of research since its beginning. At Jülich, he is now pursuing a new idea.
3.8 per cent – the efficiency that Japanese researchers achieved with their solar cell in 2009 did not knock anybody’s socks off. This value indicates how effectively solar energy is converted into solar power. Conventional solar cells made of crystalline silicon achieved up to 26 per cent in the laboratory at that time.
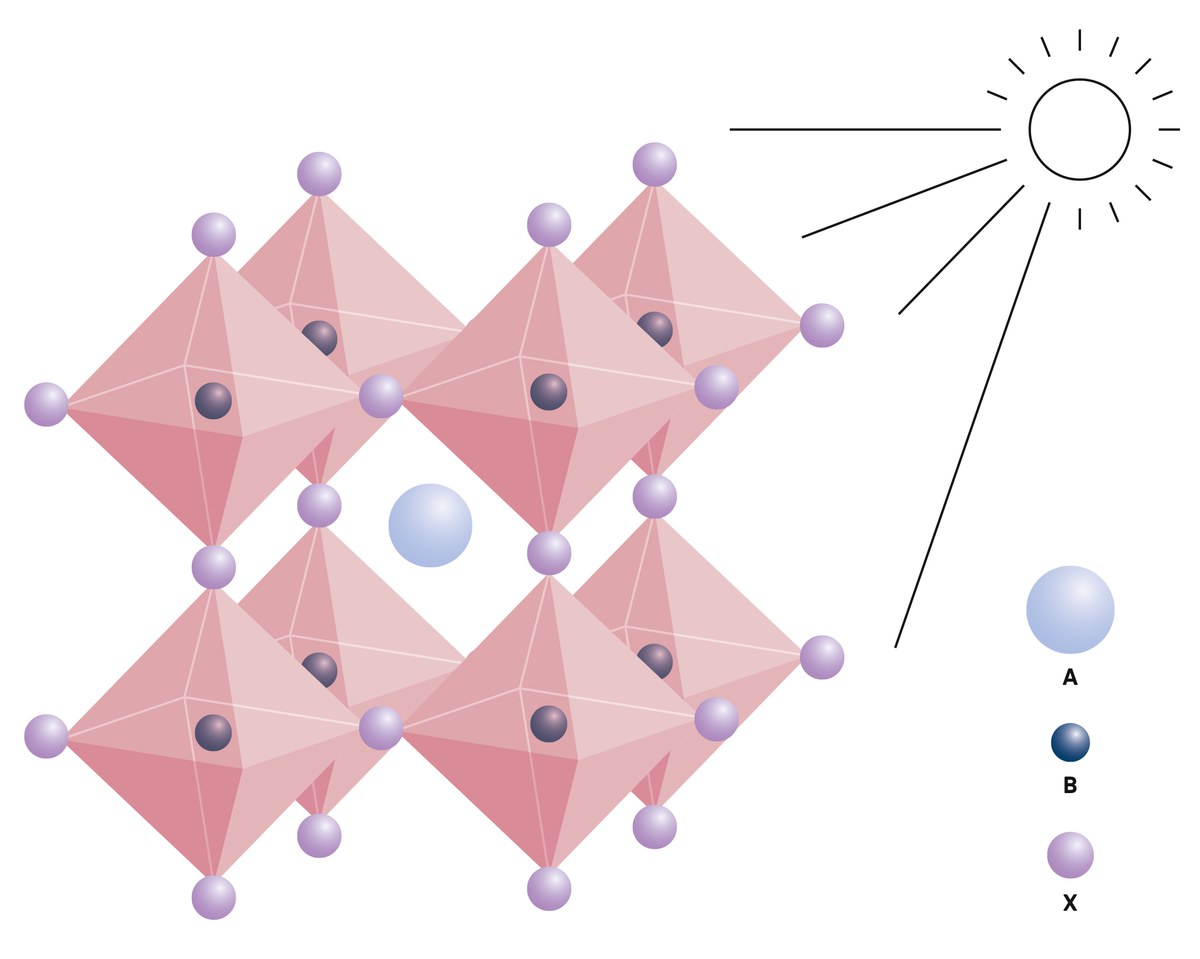
Even so, it made experts around the world perk up: the Japanese solar cell consisted of a material that had been known for 40 years and that researchers had not credited with any special properties for photovoltaics until then. Its crystal structure is characteristic for the organometallic material (see illustration). It belongs to the perovskites, named after a naturally occurring mineral. “It came as a surprise to many that this perovskite material can be a semiconductor, making it suitable for solar cells,” says Saliba. He had already started researching these materials in early 2011 as a young doctoral researcher in Oxford. Perovskites have decisive advantages over silicon crystals: they can be produced simply, cost-effectively and in an energy-saving manner.

It soon became apparent that the efficiency of perovskite solar cells could be improved enormously. A kind of gold rush broke out among materials researchers worldwide: there were already about 50 scientific publications on semiconducting perovskites in 2013 and over 3,800 in 2019. “By now, the efficiency record of perovskite solar cells is at an astonishing 25.2 per cent, which is very close to the record value of 26.7 per cent for silicon cells optimized over decades. Originally it seemed ambitious to expect that perovskites would achieve an efficiency beyond 20 per cent within just ten years. The development is unprecedented in solar cell materials research,” says Saliba.
The 37-year-old physicist is one of the stars of the research field: in 2020, he received the Heinz Maier-Leibnitz Prize of the German Research Foundation, which is one of the most important awards for young scientists in Germany. The magazine “Capital” voted him among the “Young Elite - the Top 40 under 40” in the Science and Society category twice; the magazine “MIT Technology Review” included him in the list of the world’s leading innovators under 35 in 2017; and the Institute for Scientific Information counts him among the most frequently cited scientists in his field. After stays in the USA, the UK and Switzerland, he is now the director of the Institute for Photovoltaics at the University of Stuttgart and also head of the Helmholtz Young Investigators Group FRONTRUNNER at the Jülich Institute of Energy and Climate Research (IEK-5).
Not yet stable enough
Saliba is always on the hunt for higher efficiency and a deeper understanding. In 2016, for example, working together with researchers from Switzerland, he showed that by inserting rubidium atoms into the perovskite structure, solar cells can be produced with an efficiency of 21.6 per cent. He is also concerned with probably the biggest hurdle on the way to a marketable perovskite solar cell: the lack of long-term stability. “If perovskite solar cells are to replace the established silicon technology, they must function for a comparable period of time,” says the physicist.
This is a high requirement, since silicon modules age quite slowly: they lose very little of their performance even after more than 20 years of practical use. What is more, the service life of newly developed modules can be reliably predicted using standardised methods. “However, these tests cannot simply be transferred to perovskite solar cells. Unlike silicon, perovskites react to strain such as temperature fluctuations, moisture, hail, light and preload,” Saliba explains. He is working with colleagues from all over the world to develop comparable tests for perovskite solar cells and to understand the ageing process in detail. His research team has also produced a perovskite solar cell with an efficiency of more than 20 per cent that loses power less quickly in the laboratory than previous perovskite cells. To this end, Saliba and his colleagues replaced previously used, temperature-sensitive methylammonium ions with a mixture of rubidium, cesium and formamidinium ions.
Saliba is convinced that science has so far only been digging at the edge of the perovskites “gold mine”: he has calculated that over 6,000 different perovskites would be possible by simply combining the components used so far. This number rises to over 14,000 if only one additional component were added. If it is then also taken into account that the quantity ratios of the components can be chosen almost arbitrarily, a nearly infinite variety of perovskites is the result. “I’m pretty sure that there are perovskites among them that are even better suited for solar cells, LEDs or sensors than the ones known so far,” says Saliba.
In Jülich, he wants to use automated processes to produce many of these semiconducting perovskites as micrometre -sized particles in a liquid, socalled colloids, in a short time. Pretests – which are also automated – will then provide initial indications of particularly promising candidates. The Jülich Young Investigators Group is also looking for perovskites that can be applied on top of silicon cells. Since perovskites and silicon absorb different spectral ranges of sunlight, they could be used in tandem to exploit a broader spectrum of light for power generation. “In this way, the competitors silicon and perovskite could become a dream team,” says Saliba with a chuckle.
Frank Frick

