Mechanism of bacterial sense of smell revealed
Jülich, 19 May 2017 – Scientists from the Moscow Institute of Physics and Technology, in collaboration with their colleagues from Forschungszentrum Jülich, the Institut de Biologie Structurale (IBS) and European Synchrotron Radiation Facility (ESRF) in Grenoble, have proposed a universal mechanism for the "sense of smell" in bacteria. This was done by obtaining the structure of the NarQ protein from Escherichia coli (E. coli) – which belongs to a universal class of sensory histidine kinases that are responsible for transmitting signals to bacteria about their environment. The paper published in Science will help us understand how bacteria "communicate" with one another and form biofilms on sterile surfaces or inside the human body.
Drugs which affect the "sense of smell" in bacteria could potentially be used as substitutes for modern antibiotics. These drugs do not kill bacteria; they simply supply them with signals that render them harmless to the human body. In theory, it would be impossible for resistance to be developed in this case.
All cells are isolated from the environment by a dense membrane which virtually no chemicals are able to pass through. This enables the cell to keep its internal chemical conditions constant and functioning correctly. However, the membrane greatly limits the exchange of information with the environment. In order to find out what is happening on the outside, a cell uses special molecular machines – proteins. The proteins that are designed for communicating with the environment very often "live" in the membrane itself or close to it, and they are responsible for transmitting signals or chemicals into or out of the cell.
The most universal mechanism bacteria use to "sense" the environment is two-component systems. Such systems consist of two proteins: a kinase, which receives the signal from outside the cell and transmits it into the cell, and a response regulator, which receives the signal inside the cell and triggers subsequent reactions.
A useful method of understanding how proteins function is to observe their structure at atomic-level accuracy. At present, most protein structures (more than 100,000) have been obtained using X-ray crystallography. This method involves observing the diffraction pattern of protein molecules ordered into a crystal lattice. However, this only gives the structure of one state of the protein, as in a photograph. If we can "photograph" the initial and final state of a process, we can make a guess as to how exactly the protein works when switching between these states.
Membrane "pistons" power a cell’s sense of smell
The authors of the study were able to obtain the structure of two states of the NarQ kinase from E. coli. This kinase "senses" the presence of nitrates in the environment and sends a corresponding signal through the cell membrane. As it turns out, the sensor is a dimer in both of its two states, i.e., two protein molecules work together to capture the nitrate. The first state is inactive – the protein is not bound to the nitrate ion and does not transmit a signal. The second state, on the other hand, is active, or signalling – in this state, the kinase transmits a signal into the cell to inform it that nitrates are present in the environment.
The protein structure in the active state was obtained for the most reliable "wild type" protein without the artificial mutations, which scientists often use to increase the stability of a protein. To obtain the structure in the inactive state, the authors mutated the site to which the nitrate binds. The stability of the protein was not affected; however, the nitrate no longer became bound to it, which gave the authors the opportunity to observe a kinase in a state of inactivity.
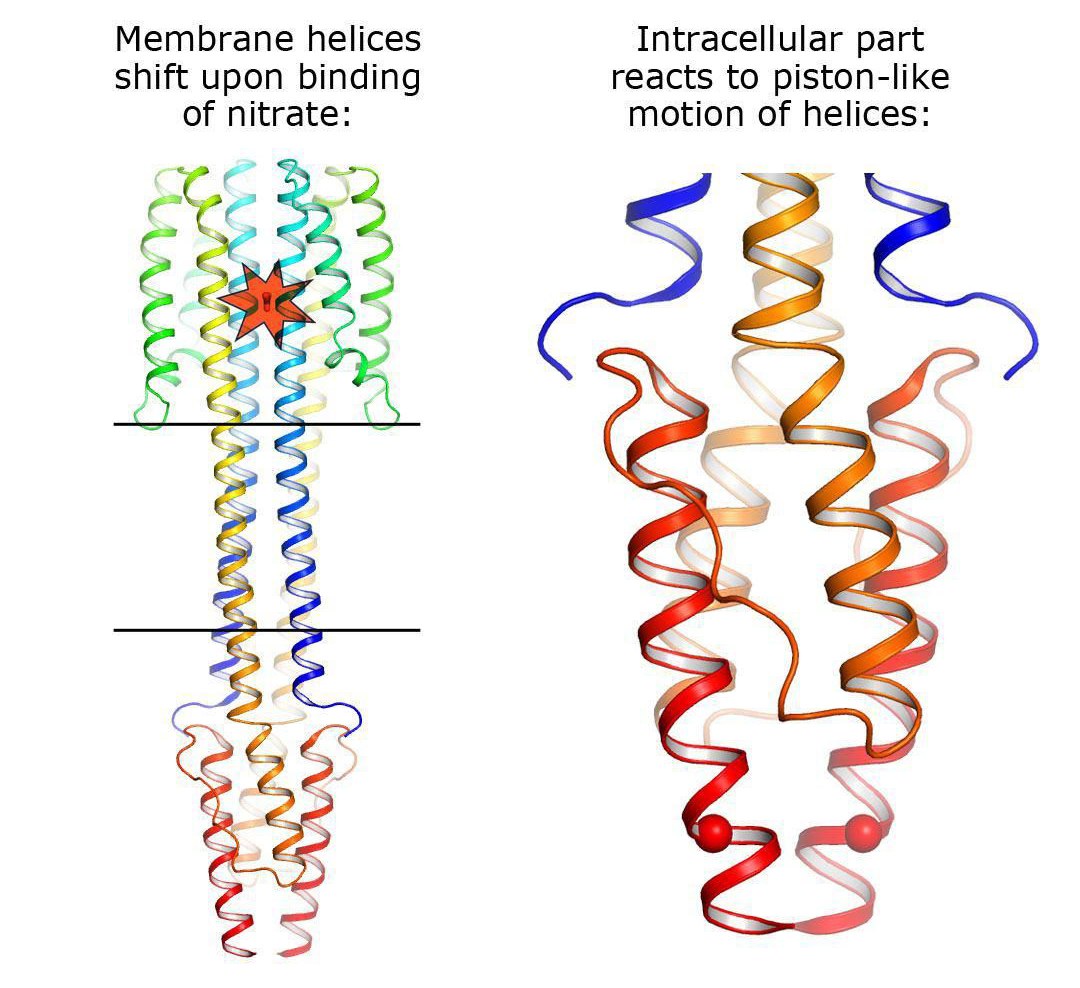
It was found that the signaling and inactive states differ only very slightly at the nitrate-binding site – by 0.5 to 1 angstroms, which is approximately one fifth of the size of the ion itself (1 angstrom is equivalent to 10-10 metres). However, when this ion binds to the sensor, it causes huge changes in the protein: The helices of different monomers begin to move in different directions, like pistons. These "pistons" transmit the small change of 0.5-1 angstroms through the membrane, and their outer ends shift in different directions by approximately 2.5 angstroms. Inside the cell, in the HAMP domain, these shifts are converted into the rotation of two parts of NarQ relative to each other. Ultimately, the positions of the output helices change by as much as 7 angstroms, thus completing the signal transmission.
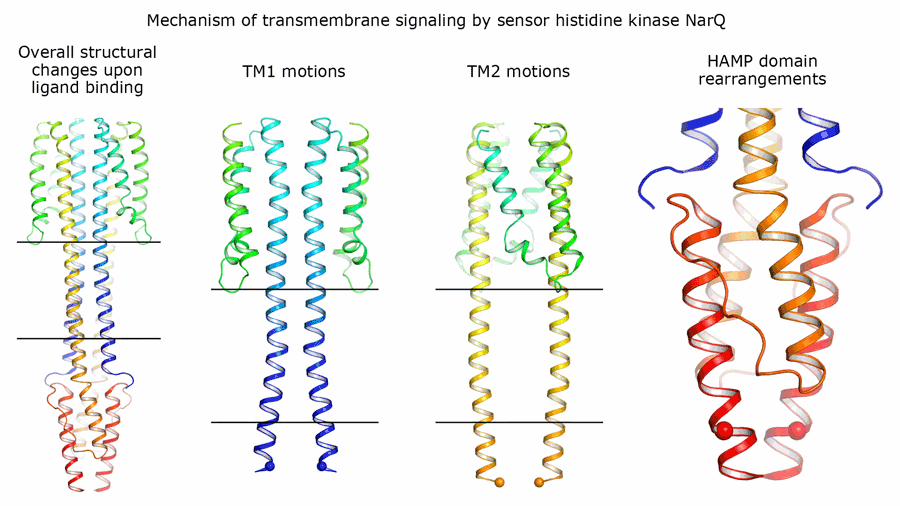
Aside from the structures in which the two proteins form a symmetrical pair, the scientists were able to produce a structure with an asymmetric position of the two proteins. In this state, the protein is "arranged" differently in the crystal and is bent strongly. However, the effect on the regulator remains virtually unchanged. This versatility of observed movement allows us to say that the signal transmission mechanism is universal, and sensors of other chemical compounds may operate via the same "piston-shift" mechanism.
"How signals are transmitted through the cell membrane is one of the most fundamental questions in modern biology. In this study, we showed in detail how a signal (in this case the binding of a nitrate) can be transmitted by hundreds of angstroms into the cells of bacteria and archaea, as well as fungi and plants. With a better understanding of the mechanisms of signal transmission, we can expect to learn how to manipulate such cells, and in particular, to try to weaken or neutralize the harmful effects of pathogenic microorganisms," said Ivan Gushchin, the Head of MIPT’s Laboratory of Structural Analysis and Engineering of Membrane Systems and, at the time of the research, also staff member at Forschungszentrum Jülich, Institute of Complex Systems: Structural Biochemistry (ICS-6), commenting on the study.
"Elucidating the structural basis of information processing in biological systems in atomic detail is a fascinating area of research", said Dieter Willbold, Director of the ICS-6. "Information gathering, integration and subsequent decision making are absolutely central processes to the phenomenon we know as life."
Original publication: Ivan Gushchin, Igor Melnikov, Vitaly Polovinkin, Andrii Ishchenko, Anastasia Yuzhakova, Pavel Buslaev, Gleb Bourenkov, Sergei Grudinin, Ekaterina Round, Taras Balandin, Valentin Borshchevskiy, Dieter Willbold, Gordon Leonard, Georg Büldt, Alexander Popov, Valentin Gordeliy
"Mechanism of transmembrane signaling by sensor histidine kinases", Science published online May 18, 2017, DOI: 10.1126/science.aah6345
Further Information:
Institute of Complex Systems, Structural Biochemistry (ICS-6)
Contact:
Dr. Ivan Gushchin
Moscow Institute of Physics and Technology
141700 Institutsky per. 9, Dolgoprudny, Russia
Tel.: +7 965 428-22-24
E-Mail: ivan.gushchin@phystech.edu
Prof. Dr. Valentin Gordeliy
Institute of Complex Systems, Strukturbiochemie (ICS-6)
Forschungszentrum Jülich
Moscow Institute of Physics and Technology
Institute de Biologie Structurale (CEA-CNRS-UJF), Grenoble
Tel.: +49 2461 61-9509
E-Mail: g.valentin@fz-juelich.de
Prof. Dieter Willbold
Institute of Complex Systems, Strukturbiochemie (ICS-6), Forschungszentrum Jülich
Institut für Physikalische Biologie der Heinrich-Heine-Universität Düsseldorf
Tel. +49 2461 61-2100
E-Mail: d.willbold@fz-juelich.de
Press contact:
Peter Zekert
Forschungszentrum Jülich
Tel.: +49 2461 61-6041 / +49 2461 61-9486
E-Mail: p.zekert@fz-juelich.de
