The Power of Less
Co-Creation – Project KernKat
Acidic water electrolysis is a promising method for producing hydrogen to support the energy transition. But it relies on the rare and expensive precious metal iridium. A new approach aims to drastically reduce the amount of iridium required.
May 2025
The right catalysts
The energy transition depends on climate-friendly alternatives to fossil fuels. This is where hydrogen comes in – particularly as an energy carrier that can power homes and factories. Hydrogen also holds potential for sustainable heavy transport and even aviation. However, producing it remains a major challenge: electrolysis – the process of splitting water into hydrogen and oxygen using electricity – requires vast amounts of energy.
“Without the right catalysts to accelerate the process and improve efficiency, water electrolysis would simply be economically unviable,” explains Prof. Simon Thiele, Head of the Department of Electrocatalytic Interface Engineering at the Helmholtz Institute Erlangen-Nuremberg (HI ERN), an external institute of Forschungszentrum Jülich.
A looming bottleneck
One of the three standard electrolysis technologies is proton exchange membrane (PEM) electrolysis, also known as acidic water electrolysis. The problem with this method: the most durable and effective catalysts contain iridium – one of the rarest metals on Earth, even rarer than gold and four to five times more expensive than platinum. It is only a byproduct of platinum mining and is extracted in just a handful of countries, including South Africa and Russia. As electrolysis scales up, the availability of elemental iridium becomes a critical bottleneck that could severely slow the development of hydrogen technology. So far, despite intensive research, nov viable alternative to iridium has been found for this application.
Reduce – don’t replace
Instead of eliminating iridium, the HoKaWe project took a different approach.
“Our goal wasn’t to replace iridium entirely, but to develop catalysts that use as little of it as possible,” says Thiele. Together with researchers from Leibniz University Hannover and companies Dyneon and Umicore, the team searched for support materials that would pair optimally with the precious metal. The result: several promising candidates identified through extensive testing.
These findings laid the groundwork for the follow-up project, KernKat. This collaborative initiative aims to answer a key question: How can we minimize the use of rare precious metals without compromising catalyst performance or durability? Partners include HI ERN, the universities of Bayreuth and Erlangen-Nuremberg, the Technical University of Munich, as well as industrial partners Umicore and Bosch.

»Without the right catalysts, water electrolysis would be economically unfeasible.«
Prof. Simon Thiele
Head of Department at the Helmholtz Institute Erlangen-Nuremberg (HI ERN)
“Our approach is quite new,” says Thiele. The idea is to use iridium more intelligently – by coating only the surface of a support material with the precious metal, while replacing the rest with a more affordable alternative. “We call these core–shell catalysts, consisting of a stable core and an ultra-thin iridium shell,” the Jülich-based researcher explains.
Ready for real-world application
As a result, catalysts based on this approach require up to 90 percent less iridium. “This means drastically reduced costs — with the same level of efficiency,” emphasizes Thiele. The team aims to go even further: they are working on core-shell-shell catalysts made of multiple nanoscale layers, which could enhance both stability and reactivity.
But even the best idea is worthless if it doesn’t work in practice. That’s why Bosch tests and evaluates the most promising catalyst solutions under real-world conditions. “Here, we’re not only developing new materials—we’re also designing them to be market-ready,” says the scientist from HI ERN.
Research focus at HI ERN: Climate-neutral energy supply of the future
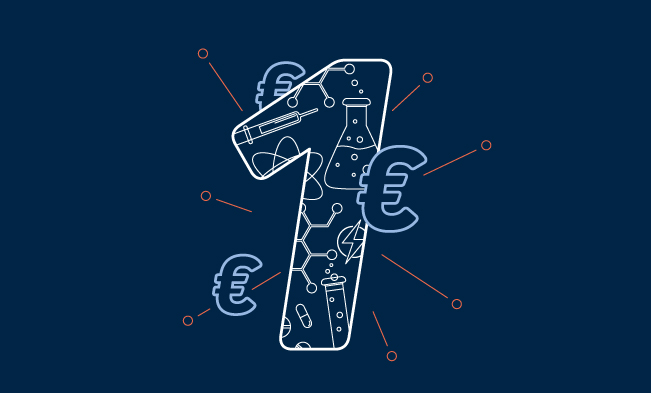
20 %
of the world’s energy demand could come from hydrogen by
2050—provided that policymakers invest and research makes it
affordable.
Source: Hydrogen Council, McKinsey, IRENA

Background and figures on the global hydrogen strategy in the IEA report
The path to industrial implementation is challenging. Long-term stability tests must demonstrate that the new catalysts continue to perform reliably even after years of use. Currently, scientists are still studying and developing the materials at the smallest scale: at the nanometer level, the future of energy could be decided. “If we succeed,” says Thiele, “we will make a fundamental contribution to the energy transition.”
Making hydrogen more affordable
Thiele’s team – around 50 researchers from chemistry, physics, and engineering – is focused not just on catalysts. “We’re researching the power-to-X-to-power technologies of tomorrow. It’s all about fundamentally improving hydrogen production,” he says. The coming decade will be critical. If hydrogen is to truly replace fossil fuels, it must become more cost-competitive. “Our goal is to make hydrogen so attractive that fossil fuels can no longer compete. Once that happens, the market will shift on its own – and that would be the real gamechanger,” says Thiele.
Research Department for Electrocatalytic Interface Process Engineering
Image credit: Giulia Iannicelli; Forschungszentrum Jülich with AI
Dive deeper into the current issue
When research, industry, and society unite their perspectives, they create solutions greater than the sum of their parts.
In the Endeavours magazine, discover how co-creation works — through real stories of collaboration, pioneering spirit, and successful transfer.