Patricia Hidalgo
Prof. Dr. Patricia Hidalgo filmt die Reise von Calcium-Ionenkanälen durch das Zellinnere.
27 November 2025
When three bright minds from different disciplines come together, something exceptional can happen. This is exactly what Prof. Patricia Hidalgo, Dr. Beatrix Santiago-Schübel, and Dr. Mercedes Alfonso-Prieto have achieved at Forschungszentrum Jülich. In an interdisciplinary project, they investigated how cells recognise and remove defective calcium channels – work that could prove significant not only for basic science, but also for future therapies for neurodegenerative diseases.

Their project vividly demonstrates the value of close interdisciplinary collaboration across institutional boundaries – and how a diverse team can benefit from different perspectives and experiences. In this interview, they share insights into their research and collaboration.
For readers who are not familiar with cell biology – what exactly are voltage-gated calcium channels, and why are they so important?
Calcium channels are tiny ‘gates’ in the cell membrane that open only when the electrical voltage across the membrane changes. Calcium ions then flow into the cell – a kind of starting signal that triggers essential processes, such as forming thoughts, recalling memories or moving muscles. This makes them a key component of how cells process information in brain and heart.
What did you discover in your joint study on calcium channels, and why is it significant for this field of research?
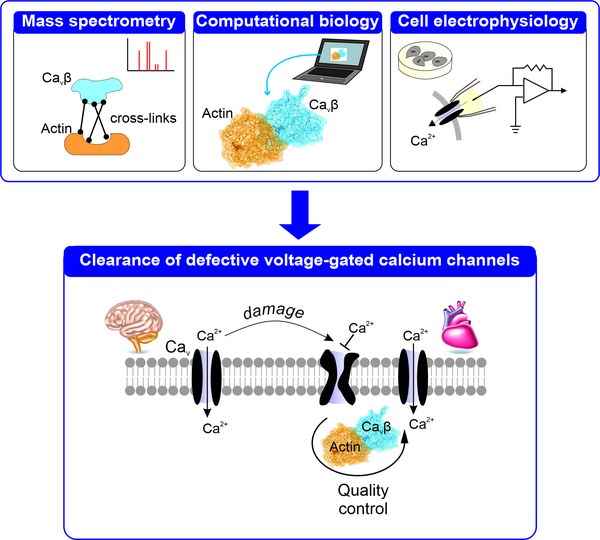
In our study, we wanted to understand how cells control the lifetime of voltage-gated calcium channels. Their lifespan is crucial because these channels govern how nerve cells communicate and how heart cells beat: if they are broken down too early or remain active for too long, the delicate balance of the system is disturbed and communication in the nervous and cardiac systems falters.
To keep cells healthy, they possess a kind of cellular quality control – a system that recognises, marks, and removes defective proteins to maintain stability and functionality. We already knew that CaVβ, a subunit of these channels, interacts with actin, a structural protein that gives cells their shape and organises internal transport.
We have now shown that this interaction is vital: if it is weakened, the number of channels on the cell surface remains unchanged, but defective channels are not being removed and replaced by healthy ones. The association between CaVβ and actin therefore helps the cell to identify defective channels and preserve the functional ones.
This finding is not only exciting from a basic research perspective, but could also have medical relevance in the future. The newly identified interface between CaVβ and actin could provide a druggable target for modulating the cell’s own quality control processes – for example, in neurodegenerative diseases or age-related functional decline, where damaged proteins accumulate.
What was the first step in this project?
The foundations were laid by Patricia at the Institute of Biological Information Processing – Molecular and Cellular Physiology, where we were able to demonstrate that CaVβ interacts directly with actin. Using patch-clamp experiments, which measure ion flow through individual channels in a cell membrane, we observed that blocking this interaction reduces calcium currents. The next logical step was to pinpoint the exact contact surface between the two proteins.
And how did you proceed from there?
Patricia actively sought collaborators across the research centre – to help her to discover the region where the two proteins interact which each other. Beatrix combined chemical cross-linking with tandem mass spectrometry, a technique that reveals precisely where proteins are linked. This enabled us to produce the first cross-linked CaVβ–actin complex and perform a detailed mass spectrometric analysis. Now, we needed someone who could translate these data into molecular distances. That is Mercedes’ area of expertise. The promising preliminary results motivated her to join the project right away. Together, we successfully applied for funding through Forschungszentrum Jülich’s “Networking Doctoral Students” programme, which enabled us to recruit Francisco Castilla from Spain as a PhD student. He worked on the project with tremendous energy and enthusiasm during the height of the Covid pandemic and encouraged his PhD colleague Victor Lugo to carry out the electrophysiological experiments.
Over several years of intensive, interdisciplinary collaboration between three institutes, we were finally able to complete the project successfully.
What do you think made this project so successful?
Our success stemmed from combining our complementary fields – electrophysiology, mass spectrometry, and computational modelling. This interdisciplinary approach allowed us to look at the same problem from different angles.
At the same time, our collaboration is a good example of the potential that diversity can unlock in research. Pursuing our aim, we became not just colleagues, but friends.
Castilla, F., Lugo, V., Miranda-Laferte, E., Jordan, N., Huesgen, P. F., Santiago-Schübel, B., Alfonso-Prieto, M., & Hidalgo, P. (2025). Mapping the interaction surface between CaVβ and actin and its role in calcium channel clearance. Nature Communications, 16(1), 4352. https://doi.org/10.1038/s41467-025-59548-x