Theory of Electrocatalytic Interfaces | Junior Prof. Dr. Jun Huang
About
We develop physics-based models for interfaces and reactions in electrocatalysis. We take a hierarchical approach towards Operando modelling of reactive electrochemical interfaces on the mesoscale.
Funded by: Helmholtz Investigator Group, ERC Starting Grant
"Insight must precede application"
(Max Planck, 1858 - 1947)
Our mission
- Contributing to fundamental understanding of interfacial electrochemistry by developing new theoretical concepts and methods, specifically,
- Developing density-potential functional theoretic schemes as a viable theoretical method to reactive, heterogeneous, dynamic electrical double layers on the mesoscale
Research Topics
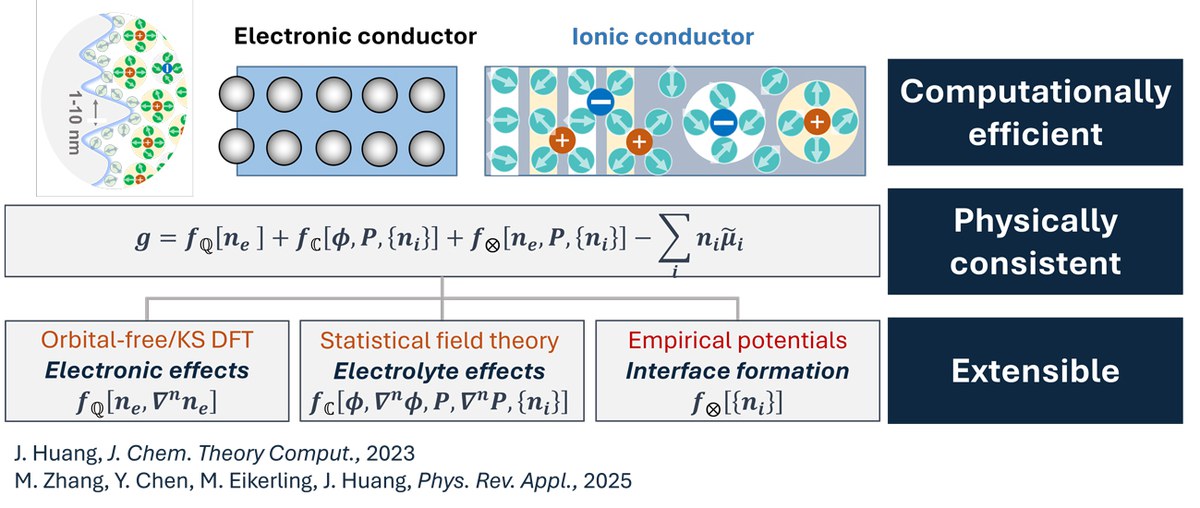
Density Potential Functional Theoretic Methods
Simulating electron transfer at reactive solid-liquid interfaces under constant electrochemical potentials of the constituents (electrons, ions, solvent etc.) is crucial to understanding the formation, functional and failure of electrochemical devices and beyond. Albeit being sufficiently accurate in decreasing breaking and formation of chemical bond at solid surfaces, existing methods based on Kohn-Sham density functional theory (DFT) are unsatisfactory in system consistency, namely, simulating the solid-liquid interface under grand-canonical conditions, as well as in scaling up the simulation due to its high computational cost. Herein, to improve the system consistency and computational efficiency, we develop density-potential functional theoretic (DPFT) schemes out of Kohn-Sham DFT, drawing upon ideas of orbital-free DFT, frozen density embedding theory, and tight-binding DFT. The DPFT transforms an all-atom, Kohn-Sham DFT description of the nonreactive electrolyte solution into a coarse-grained, field-based description, while retaining a Kohn-Sham DFT description for the reactive subsystem. In the absence of surface reactions, the solid electrode can be described using orbital-free DFT, reducing the computational cost further.
Theory of Electron Transfer in EDL
Electrocatalytic reactions occur in the electrical double layer (EDL) at the electrode-electrolyte interfaces, while the influence of the EDL structure on the reaction is unclear even for simplest reactions. We aim at developing fundamental understanding of EDL effects on elementary charge transfer at electrocatalytic interfaces, using model Hamiltonian approaches under both equilibrium and quantum dynamics regimes. The focus is put on electron transfer at highly charged electrode surfaces, like the Ag electrode in carbon dioxide reduction reaction, for which the classical EDL models and electron transfer theories might be inadequate.
Heterogeneous, Mesoscopic, Dynamic Interfaces
Real-world electrocatalysts are often heterogeneous in composition on the mesoscopic scale, while most fundamental understanding has been collected on ideally planar electrodes. A prominent example is Pt nanoparticles supported on carbon materials, featuring the overlap of the EDLs of Pt and carbon. In addition, these reactive interfaces are not static but evolve dynamically during operation. It is our long-term goal to understand how the mesoscopic heterogeneities influence the EDL structure and reaction kinetics, and in turn how the reactions drive the dynamic evolution of the interfaces.
"Interest → Preparation → Breakthrough,
I think, is the necessary three-step process for any research work”
(Chen-Ning Yang, 1922-)
Members
Visitors
Alumni
"Every mathematician has only a few tricks"
(Gian-Carlo Rota, 1932-1999)