Casey Paquola receives Early Excellence in Science Award
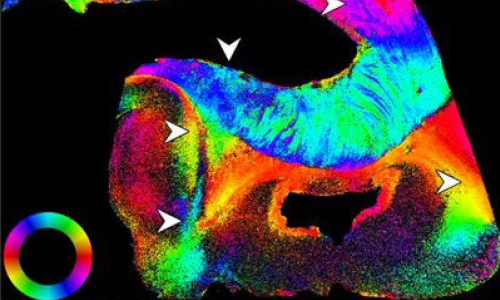
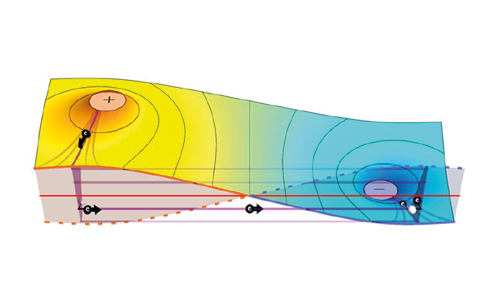
Dr Casey Paquola from Forschungszentrum Jülich has been honoured with the Early Excellence in Science Award by the Bayer Foundation. The neuroscientist received the award in the category of Biology for her research on simulating human brain maturation – work that could help to detect abnormalities in brain development at an earlier stage. The award ceremony will take place in Leverkusen in February 2026.